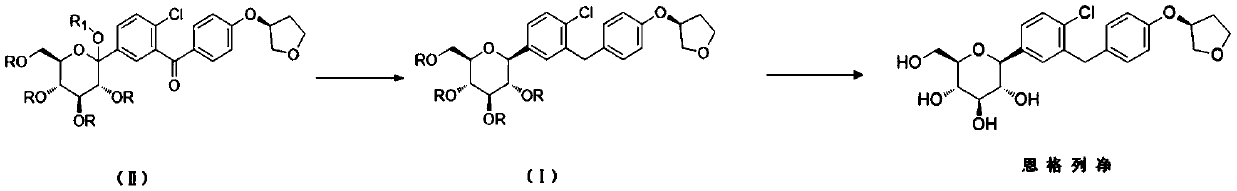
Synthesizing method suitable for industrial empagliflozin production
A synthetic method, the technology of empagliflozin, applied in the field of industrial production of empagliflozin, can solve the problems of difficult refining and purification of intermediates, cumbersome operation, high process cost, etc., achieve strong practical application value and improve product quality , reduce the effect of high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
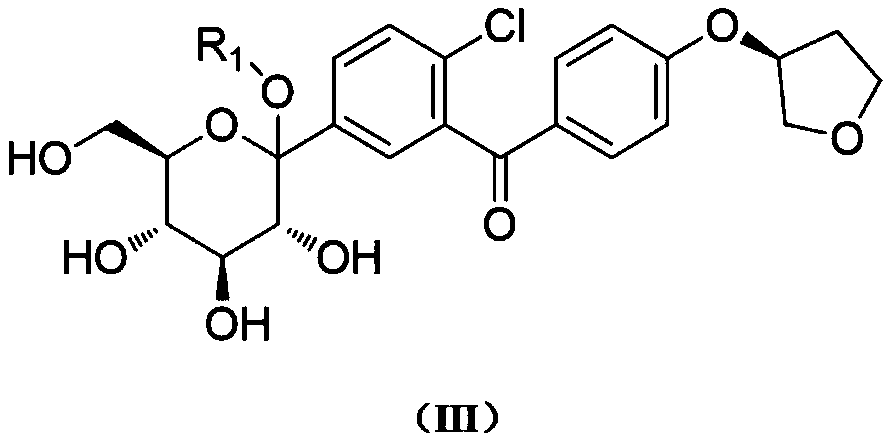
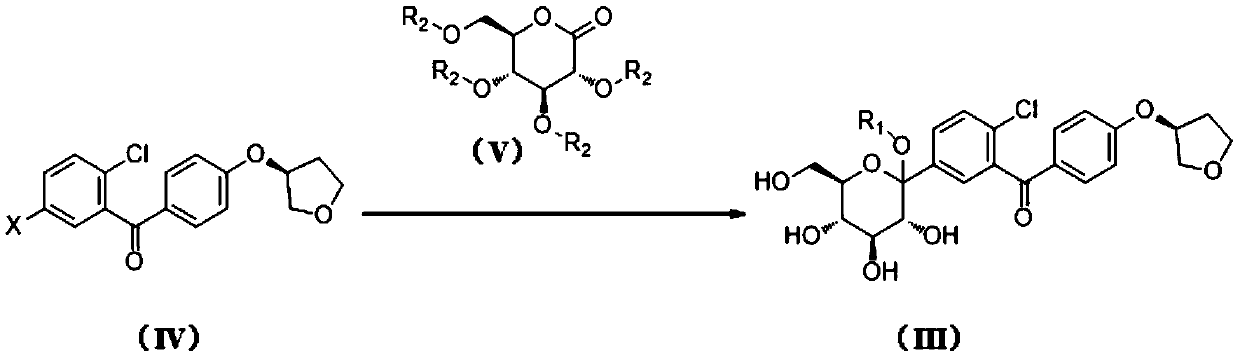
[0042] Step (1): Preparation of compound (III-1)
[0043] Add 117 g of compound (IV-1) and a mixed solvent of toluene / tetrahydrofuran (volume ratio 2:1, total volume 700 mL) into a four-neck flask, stir and mix evenly. Under the protection of nitrogen, the internal temperature of the system was lowered to -80° C., and then 93 g of a 2.5 M n-butyllithium solution in n-hexane was added dropwise. After the addition was completed, 300 g of compound (V-1) in toluene was added dropwise after stirring for 20 minutes (150 g of compound (V-1) was diluted with an equal amount of toluene). After the addition, keep stirring for 2 hours. Continue to dropwise add methanesulfonic acid / methanol mixed solution (59g / 230g). After the addition was complete, the temperature of the system was raised to 30°C and the reaction was stirred for 12 hours. After the reaction was finished, the reaction solution was added to 500ml of saturated aqueous sodium bicarbonate solution to quench, and then 1000m...
Embodiment 2
[0051] Step (1): Preparation of compound (III-1)
[0052] Add 8.0Kg of compound (IV-1) and a mixed solvent of toluene / tetrahydrofuran (volume ratio 2:1, total volume 48.0L) into a 200L ultra-low temperature reactor, stir and mix well. Under the protection of nitrogen, the internal temperature of the system was lowered to -80°C, and then 6.4Kg of 2.5M n-butyl lithium in n-hexane was added dropwise. After the addition was completed, 20.6 kg of compound (V-1) in toluene was added dropwise after stirring for 30 minutes (10.3 kg of compound (V-1) was diluted with an equal amount of toluene). After the addition, keep stirring for 2 hours. Continue to drop methanesulfonic acid / methanol mixed solution (4.0Kg / 15.7Kg). After the addition was complete, the temperature of the system was raised to 30°C and the reaction was stirred for 12 hours. After the reaction was completed, the reaction solution was added to 34.2Kg saturated aqueous sodium bicarbonate solution to quench, and then 54...
Embodiment 3
[0060] Step (1): Preparation of compound (III-1)
[0061] Add 20.0Kg of compound (IV-1) and a mixed solvent of toluene / tetrahydrofuran (volume ratio 2:1, total volume about 120L) into a 500L ultra-low temperature reactor, stir and mix well. Under the protection of nitrogen, the internal temperature of the system was lowered to -80°C, and then 15.9Kg of 2.5M n-butyl lithium in n-hexane was added dropwise. After the addition was completed, 51.2 kg of compound (V-1) in toluene was added dropwise after stirring for 30 minutes (25.6 kg of compound (V-1) was diluted with an equal amount of toluene). After the addition, keep stirring for 2 hours. Continue to drop methanesulfonic acid / methanol mixed solution (10.0Kg / 39.3Kg). After the addition was complete, the temperature of the system was raised to 30°C and the reaction was stirred for 12 hours. After the reaction, the reaction solution was added to 85.5Kg saturated aqueous sodium bicarbonate solution to quench, then 140Kg ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com