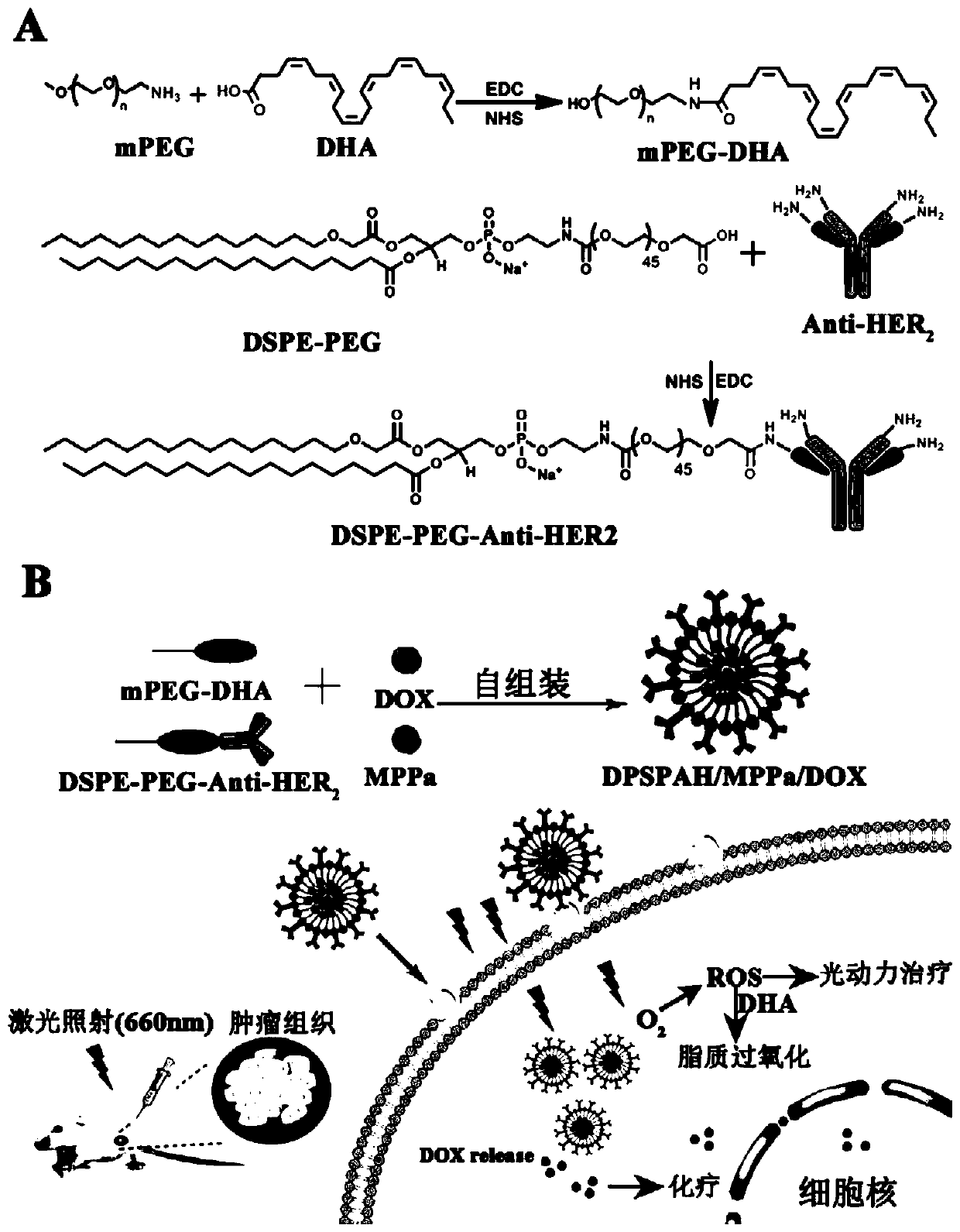
Preparation and application of tumor-targeting drug based on unsaturated fatty acid nanoparticles
A nanoparticle, carboxyl technology, applied in the field of nanomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] The synthesis of embodiment 1 nanoparticle DPSPAH / MPPa / DOX
[0124] 1. Preparation of mPEG-DHA chain.
[0125] Utilize EDC / NHS to activate the carboxyl group of unsaturated fatty acid (DHA) in solvent DMF, and then react with amino polyethylene glycol (mPEG-NH 2 ) condensation reaction of amino groups to form mPEG-DHA chains.
[0126] Specific method: Dissolve 100 μmol (19.17 mg) ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) in 1 mL dimethylformamide (DMF), 100 μmol (11.509 mg) hydroxyl Thiosuccinimide (NHS) was dissolved in 1 mL of dimethylformamide (DMF), 20 μmol (6.57 mg) of docosahexaenoic acid (DHA) was first dissolved in 1 mL of dimethyl sulfoxide, and then added 3mL of DMF, then mix the DHA solution and the EDC / NHS solution, stir at room temperature for 4h, then add 40μmol (80mg) mPEG-NH, a hydrophilic substance containing amino groups 2 , stirred at room temperature for 24h. Finally, it was dialyzed in PBS (pH7.4), and then freeze-dried with...
Embodiment 2
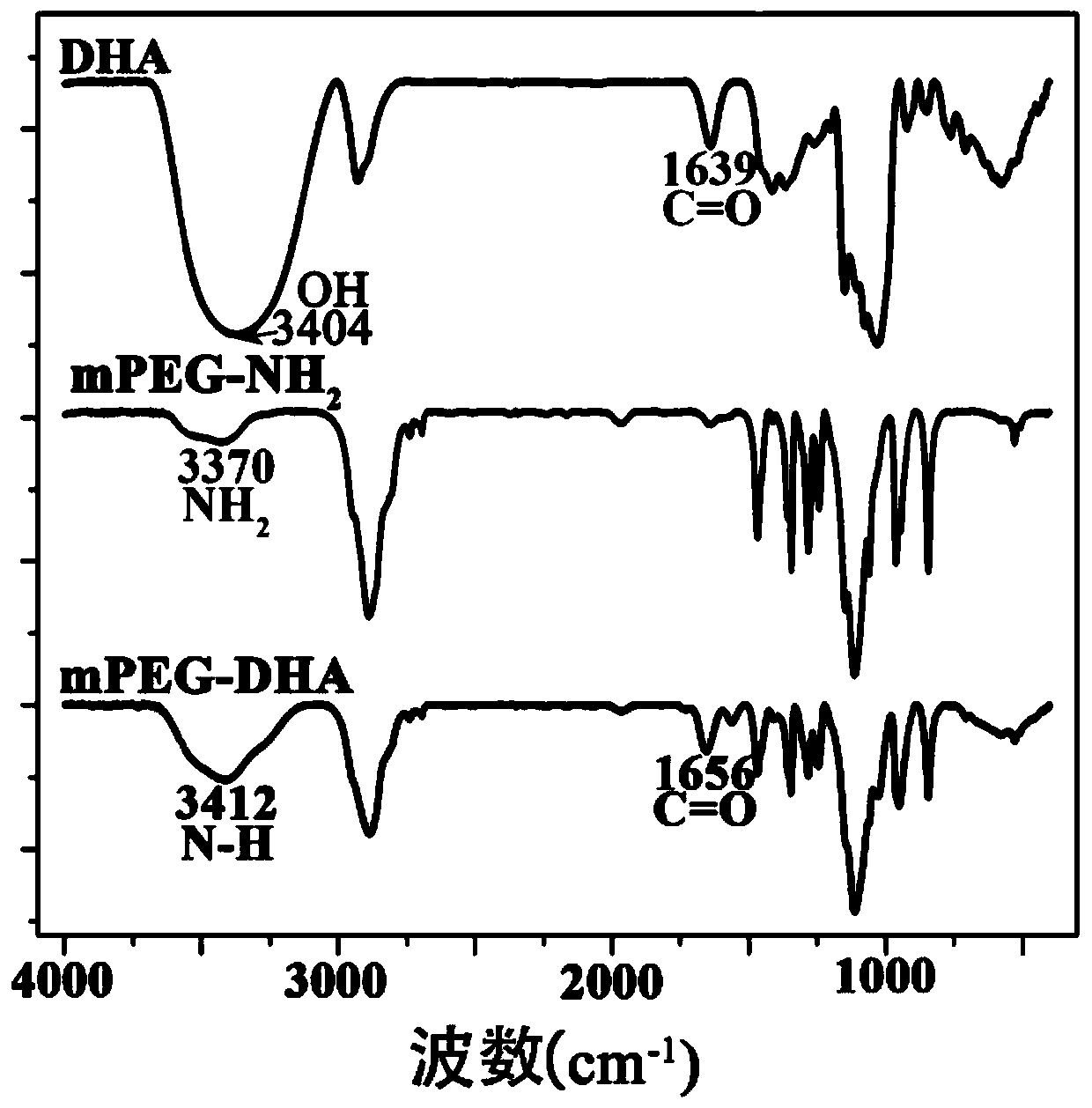
[0137] Example 2 Micellar mPEG-DHA, Micellar DSPE-PEG-Anti-HER 2 and characterization of nanoparticles
[0138] 1. Infrared spectrum detection
[0139] 1. Experimental method
[0140] In the infrared detection process, we mainly detect the formation of amide bonds. Specific detection method: Make the prepared micellar mPEG-DHA into a solid with a vacuum freeze dryer, take about 3 mg, and take about 3 mg of amino PEG and DHA respectively. The sample must be dried in a 40°C oven before infrared detection. Dry the dried sample and KBr in a dryer, mix 1-2 mg of the sample to be tested with 200 mg of pure KBr and grind them evenly, and grind the mixture to a particle size of less than 2 μm to avoid the influence of scattered light. The mixture is placed in a mold, and the mixture is pressed into a transparent sheet with a pressure of 5-10 MPa on a hydraulic press, and measured on the machine.
[0141] 2. Experimental results
[0142] The result is as figure 2 Shown, the car...
Embodiment 3
[0188] Example 3 Nanoparticles inhibit breast cancer in vitro
[0189] 1. Morphological observation
[0190] 1. Experimental method
[0191] Take out the cultured cells from the incubator, digest with trypsin for 2min, add 4mL culture medium and blow the cells. Add 3mL of cell suspension to a centrifuge tube, add 1mL of culture medium, and blow the cells to make the cells evenly suspended. Use a pipette gun to draw 100 μL of the cell suspension and add it to a 24-well plate to allow the cells to adhere to the wall and culture for 6 hours. Take out the synthesized nano drug-loaded micelles under light-shielded conditions, shake slightly and shake well. Take out the cultured cells, suck off the cell waste liquid, add PBS to wash twice, and add fresh medium. Then add the nanoparticles DPSPAH / MPPa / DOX prepared in Example 1, incubate in the dark for 4h, and irradiate laser light (660nm, 100mW / cm 2 , 10min). After culturing in the incubator for 0h, 6h, 12h and 24h, photographs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com