Composite catalyst, preparation method thereof and preparation method of ethylene
A composite catalyst and catalyst technology, applied in the direction of catalyst activation/preparation, carbon compound catalyst, catalyst, etc., can solve the problem of low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
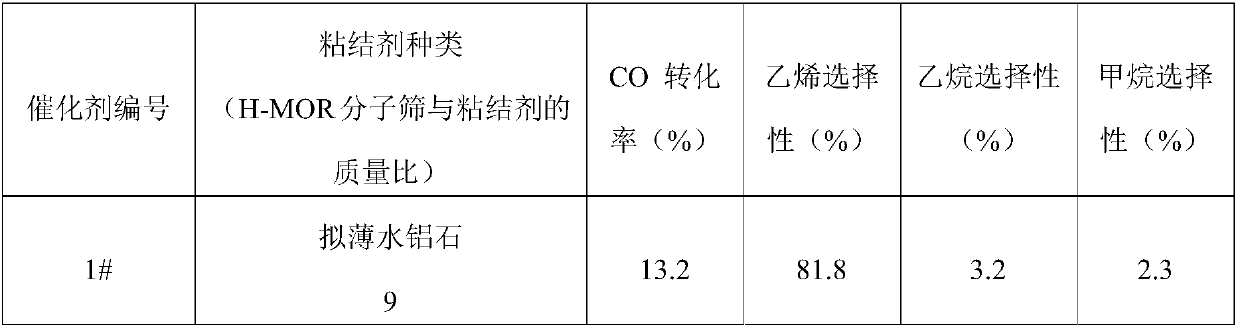
[0121] Weigh 21.46g Zr(NO 3 ) 4 ·5H 2 O, 11.90g Zn(NO 3 ) 2 ·6H 2 O and 7.5g Al(NO 3 ) 3 9H 2 O in a beaker, add 150mL deionized water, stir to obtain salt solution A. Weigh 23.55 g of ammonium carbonate into a beaker, add 150 mL of deionized water, and stir thoroughly to obtain precipitant alkali solution B. Under the condition of vigorous stirring (stirring rate is 450rpm / min), the salt solution A and the precipitant alkaline solution B are mixed in parallel flow, and the relative flow rate of solutions A and B is adjusted to ensure that the pH of the precipitation mixture is kept between 7 and 8 between. After co-precipitation, aging for 2h. After that, it was dried in an oven at 100° C. for 6 hours, and calcined in a muffle furnace at 500° C. for 4 hours to obtain a methanol synthesis catalyst. According to XRF elemental analysis, the methanol synthesis catalyst is composed of (ZnO) 0.4 (ZrO 2 ) 0.5 (Al 2 o 3 ) 0.1 .
[0122] Select pseudo-boehmite respec...
Embodiment 2
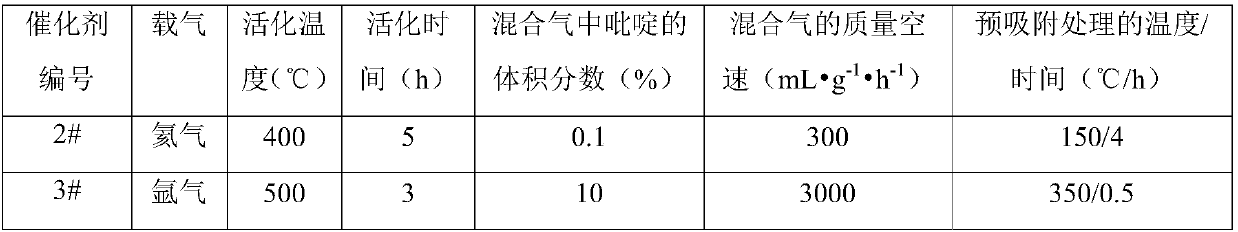
[0129]The methanol synthesis catalyst was obtained by using the same preparation method and preparation conditions as in Example 1. The specific preparation conditions of the modified H-MOR molecular sieve are shown in Table 2 below, and the rest of the operations are the same as in Example 1. The method and conditions for preparing the composite catalyst from the methanol synthesis catalyst and the modified H-MOR molecular sieve are the same as in Example 1.
[0130] Table 2
[0131] Catalyst number Calcination temperature (℃) Roasting time (h) Mass ratio of H-MOR molecular sieve to binder 2# 550 2 0.1 / 1 3# 400 6 6 / 1
[0132] table 3
[0133]
[0134] Catalyst 4#: The difference from Example 1 is that the carrier gas during the preparation of the modified H-MOR molecular sieve is CO 2 .
[0135] Catalyst 5#: The difference from Example 1 is that the carrier gas is hydrogen during the preparation of the modified H-MOR molecular sieve.
...
Embodiment 3
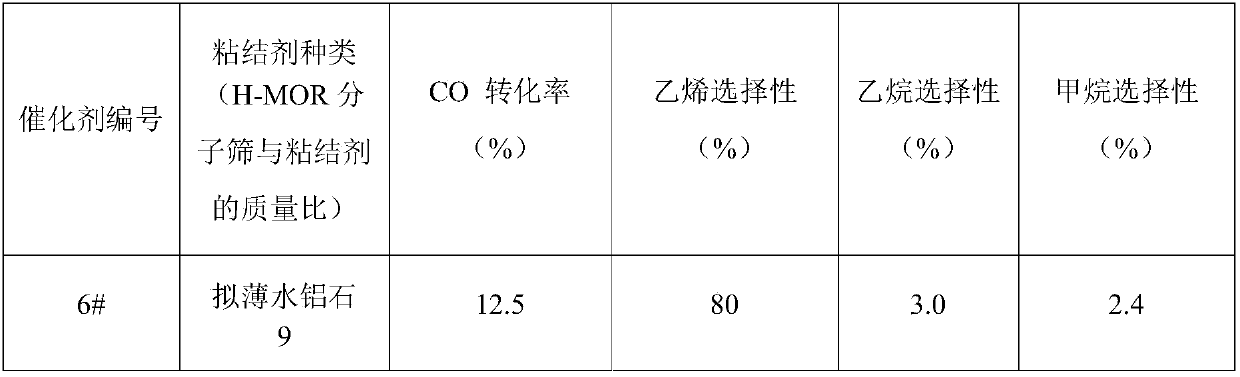
[0138] Adopt impregnation method to prepare methanol synthesis catalyst, concrete steps are as follows: Weigh 11.90g Zn(NO 3 ) 2 ·6H 2 O in a beaker, add 150mL of deionized water, stir to obtain salt solution C, 7.6g of chromium oxide powder and 2.04g of aluminum oxide are immersed in solution C, after immersion for 5h, slowly evaporate the solvent to dryness, and after preliminary drying, place in an oven Dry at 100°C for 10 hours. The dried solid powder was calcined at a temperature range of 550°C for 4h. Obtain methanol synthesis catalyst, its composition is (ZnO) 0.4 (Cr 2 o 3 ) 0.5 (Al 2 o 3 ) 0.1 .
[0139] Except that the preparation method of the methanol synthesis catalyst is different from that of Example 1, the rest of the steps are consistent with that of Example 1, and the catalyst obtained at last is marked as 6#. Under the same reaction conditions as in Example 1, the 6# catalyst was evaluated, and the reaction product was analyzed online by a gas chr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com