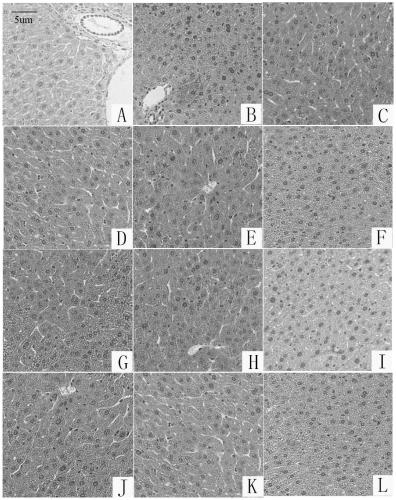
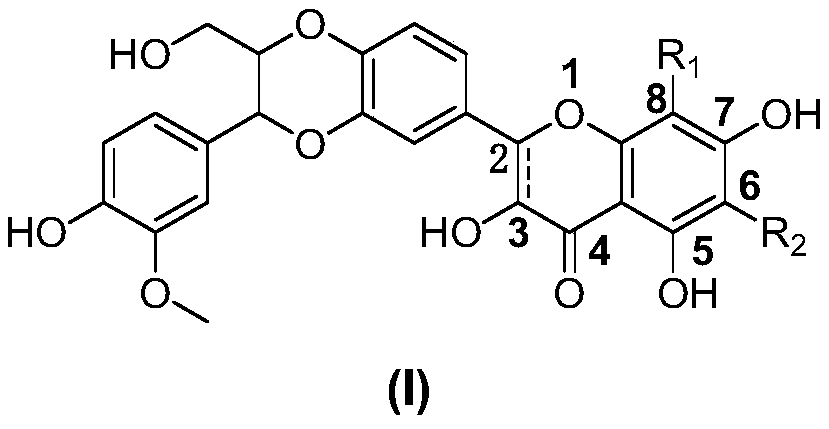
Aminomethyl substituted silybin derivative and preparation method and application thereof
A technology of silibinin and dehydrosilibinin, which is applied in the field of compounds, can solve the problems of not synthesizing aminomethylated derivatives, etc., and achieve the effects of improving bioavailability, protecting liver damage, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
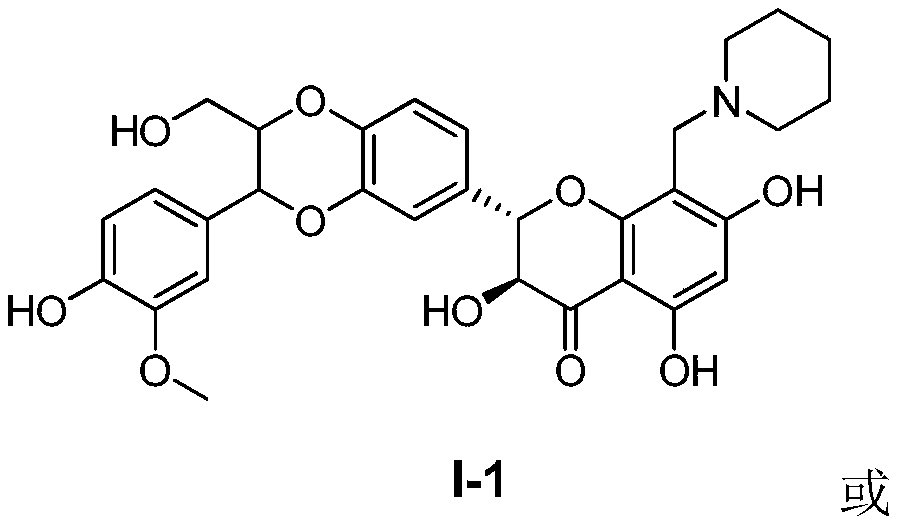
[0059] Embodiment 1: Preparation of 8-(N-methylenepiperidinyl) silibinin (I-1) and its hydrochloride
[0060]
[0061] Ⅰ-1 hydrochloride
[0062]
[0063] Put silibinin (5.0 g, 10.4 mmol), 37% aqueous formaldehyde (0.48 g, 5.2 mmol), and piperidine (0.44 g, 5.2 mmol) in 50 mL of methanol and stir at room temperature. TLC (dichloromethane:methanol=10:1) tracked the reaction, and the reaction was complete within 24 hours, and the reaction was terminated. Spin-dry under reduced pressure to obtain 5.7 g of crude product, add 100 ml of ethyl acetate to make slurry for 2 h, filter under reduced pressure, and separate by silica gel column chromatography (CH2Cl2-CH3OH=10:1-8:1) to obtain 0.9 g of (I-1) (yield rate 30%). The prepared (I-1) was dissolved in methanol-hydrochloric acid solution (100ml), stirred for 0.5h, concentrated and dried to obtain 0.95g of (I-1) hydrochloride. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.02~6.82(6H,m), 5.62(1H,s), 4.99~4.94(1H,m), 4.93~4.90(1H,m), 4.56...
Embodiment 2
[0064] Embodiment 2: Preparation of 6,8-bis(N-methylenepiperidinyl) silibinin (I-2) and its hydrochloride
[0065]
[0066] I-2 hydrochloride
[0067]
[0068] Silibinin (5.0g, 10.4mmol), 37% formaldehyde aqueous solution (3.38g, 41.7mmol), piperidine (3.55g, 41.7mmol) were placed in 25mL methanol, stirred at room temperature, TLC (dichloromethane: methanol =10:1) Follow up the reaction, complete the reaction within 24 hours, and terminate the reaction. Spin-dried under reduced pressure to obtain 6.5 g of the crude product, which was slurried with 100 ml of ethanol for 2 hours, then filtered under reduced pressure, and the solid was dried to obtain 3.7 g of (I-2) (yield 52.9%). The prepared (I-2) was dissolved in methanol-hydrochloric acid solution (100ml), stirred for 0.5h, concentrated and dried to obtain 3.9g of (I-2) hydrochloride. 1H NMR(400MHz,DMSO-d6)δ7.12-6.80(6H,m), 4.99-4.94(1H,m), 4.93-4.90(1H,m), 4.66-4.46(1H,m), 4.18-4.15 (1H,m), 3.78(3H,s), 3.60(4H,m), 3...
Embodiment 3
[0069] Embodiment 3: Preparation of 8-(N-methylenediethylamino) silibinin (I-3) and its hydrochloride
[0070]
[0071] I-3 hydrochloride
[0072]
[0073] Put silibinin (10.0 g, 20.8 mmol), 37% formaldehyde aqueous solution (1.0 g, 10.4 mmol), diethylamine (0.76 g, 10.4 mmol) in 80 mL of methanol, and stir at room temperature. TLC (dichloromethane:methanol=10:1) tracked the reaction, and the reaction was complete within 24 hours, and the reaction was terminated. The crude product was spin-dried under reduced pressure to obtain 10.3 g, which was slurried by adding 100 mL of ethyl acetate for 2 h, filtered under reduced pressure, and separated by silica gel column chromatography (CH2Cl2-CH3OH=10:1-8:1) to obtain 2.1 g of (I-3) (yield rate 19%). The prepared (I-3) was dissolved in methanol-hydrochloric acid solution (100 mL), stirred for 0.5 h, concentrated and dried to obtain 2.12 g of (I-3) hydrochloride. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.07~6.82(6H,m), 5.54(1H,s), 4.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com