1,2-dipalmitoyl-SN-glycerol-3-phosphoethanolamine and preparation method thereof
A technology of phosphoethanolamine and dipalmitin, which is applied in the field of compound preparation, can solve problems such as difficult removal, large polar impurities, and poor purity of the final product, and achieve less by-products, high selectivity, and high overall yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
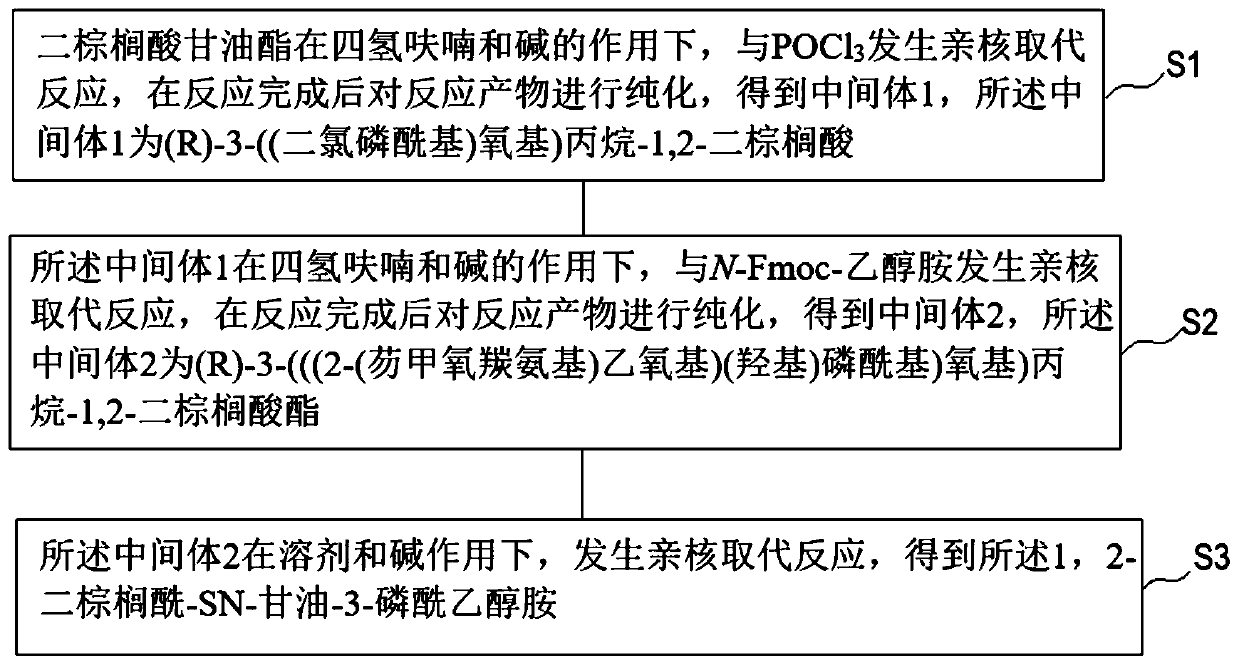
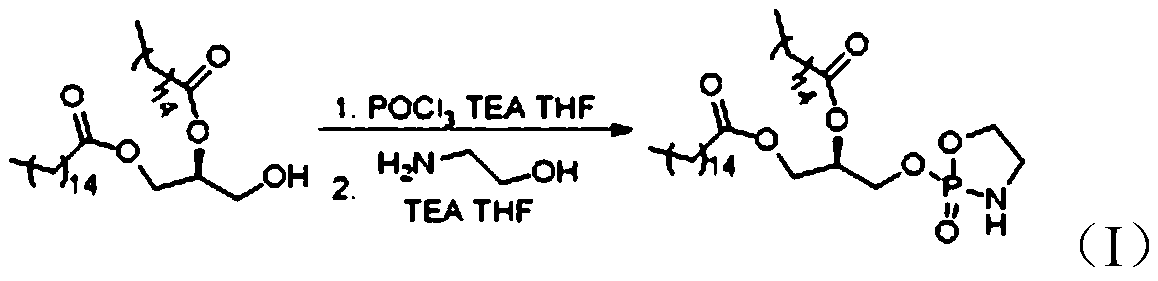
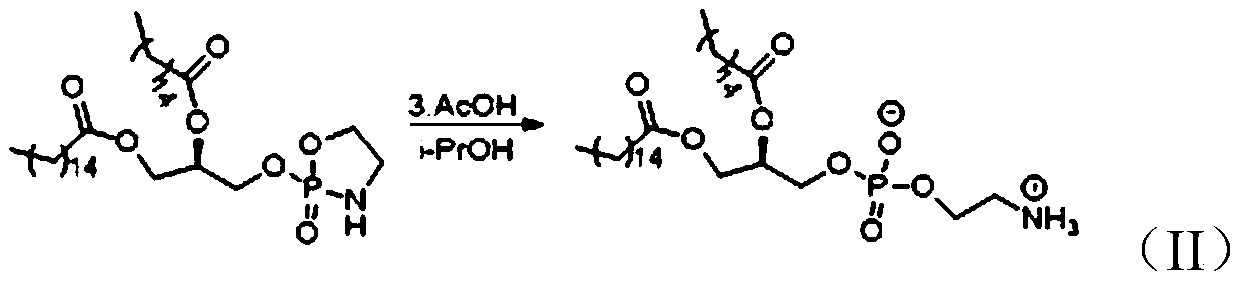
[0031] Such as figure 1 As shown, the preparation method of 1,2-dipalmitoyl-SN-glycerol-3-phosphoethanolamine according to the embodiment of the present invention includes the following steps:
[0032] Step S1, the dipalmitic acid glyceride under the action of tetrahydrofuran (THF) and alkali, and POCl 3 A nucleophilic substitution reaction occurs. After the reaction is completed, the reaction product is purified to obtain Intermediate 1, which is (R)-3-((dichlorophosphoryl)oxy)propane-1,2-di Palmitate.
[0033] Wherein, the base may be triethylamine (TEA), diisopropylethylamine (DIEA), pyridine, or a mixture thereof.
[0034] For example, as shown in the following formula (III), dipalmitic acid glyceride under the action of tetrahydrofuran and triethylamine, and POCl 3 A nucleophilic substitution reaction takes place to obtain intermediate 1 (ie (R)-3-((dichlorophosphoryl)oxy)propane-1,2-dipalmitate)
[0035]
[0036] Preferably, the dipalmitic acid glyceride: alkali: POCl 3 The mol...
example 1
[0055] In a 50ml round bottom flask, dissolve 3g dipalmitate in 10mL tetrahydrofuran, add 0.8g TEA; add 1.21g POCl 3 Dissolve in 20mL tetrahydrofuran, drip into the reaction system under ice water bath, keep the ice water bath for reaction. TLC monitors that the reaction is complete, the insoluble solid is removed by suction filtration, the mother liquor is evaporated to dryness under reduced pressure, 20 mL of acetonitrile is added to make a slurry, and 3 g of white solid is obtained by suction filtration, with a yield of 83%.
[0056] The reaction product was subjected to nuclear magnetic resonance experiments to confirm the structure of the product. The data are as follows:
[0057] 1 H NMR(500MHz, CDCl 3 )δ5.22--5.38(m,1H), 4.41-4.58(m,1H), 4.35-4.38(m,1H), 4.08-4.29(m,2H), 2.30-2.44(m,4H), 1.62- 1.67 (m, 4H), 1.14-1.45 (m, 48H), 0.90 (t, J = 6.9 Hz, 6H). The test result is consistent with the literature value.
example 2
[0059] In a 50ml round bottom flask, dissolve 3g dipalmitate glyceride in 10mL tetrahydrofuran, add 1g DIEA; add 1.21g POCl 3 Dissolve in 20mL tetrahydrofuran, drip into the reaction system under ice water bath, keep the ice water bath for reaction. TLC monitors the completion of the reaction, removes the insoluble solids by suction filtration, evaporates the mother liquor to dryness under reduced pressure, adds 20 mL of acetonitrile to make a slurry, and obtains 2.2 g of white solids by suction filtration. The yield is 61%.
[0060] The reaction product was subjected to nuclear magnetic resonance experiments to confirm the structure of the product. The data are as follows:
[0061] 1 H NMR(500MHz, CDCl 3 )δ5.22--5.38(m,1H), 4.41-4.58(m,1H), 4.35-4.38(m,1H), 4.08-4.29(m,2H), 2.30-2.44(m,4H), 1.62- 1.67 (m, 4H), 1.14-1.45 (m, 48H), 0.90 (t, J = 6.9 Hz, 6H). The test result is consistent with the literature value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com