Method for preparing coumarin fluorescent agent through copper acetate catalysis
A technology for catalyzing preparation of coumarins, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of many reaction steps, low reaction efficiency, and limited scope of application of the method, and achieves mild synthesis conditions, High chemoselectivity and controllable chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of 3a product
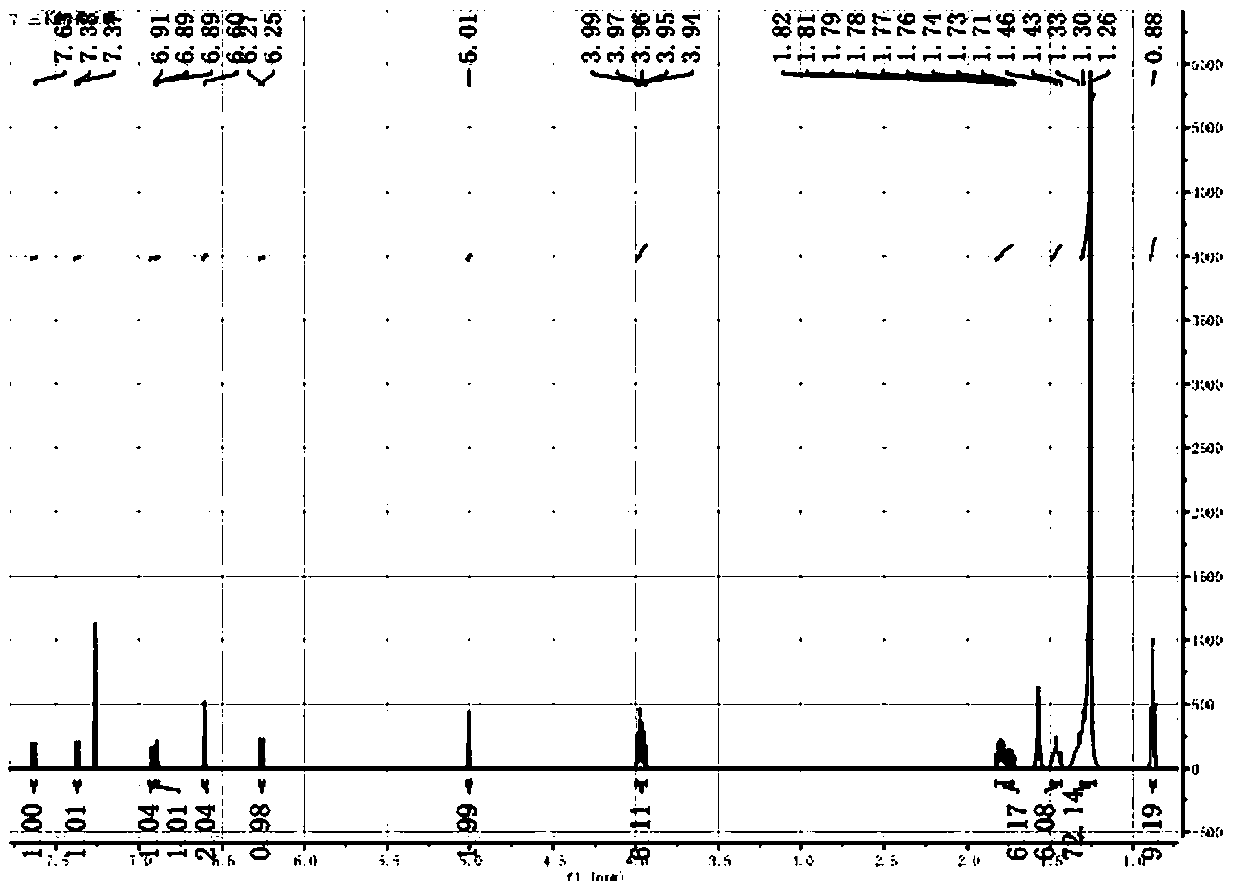
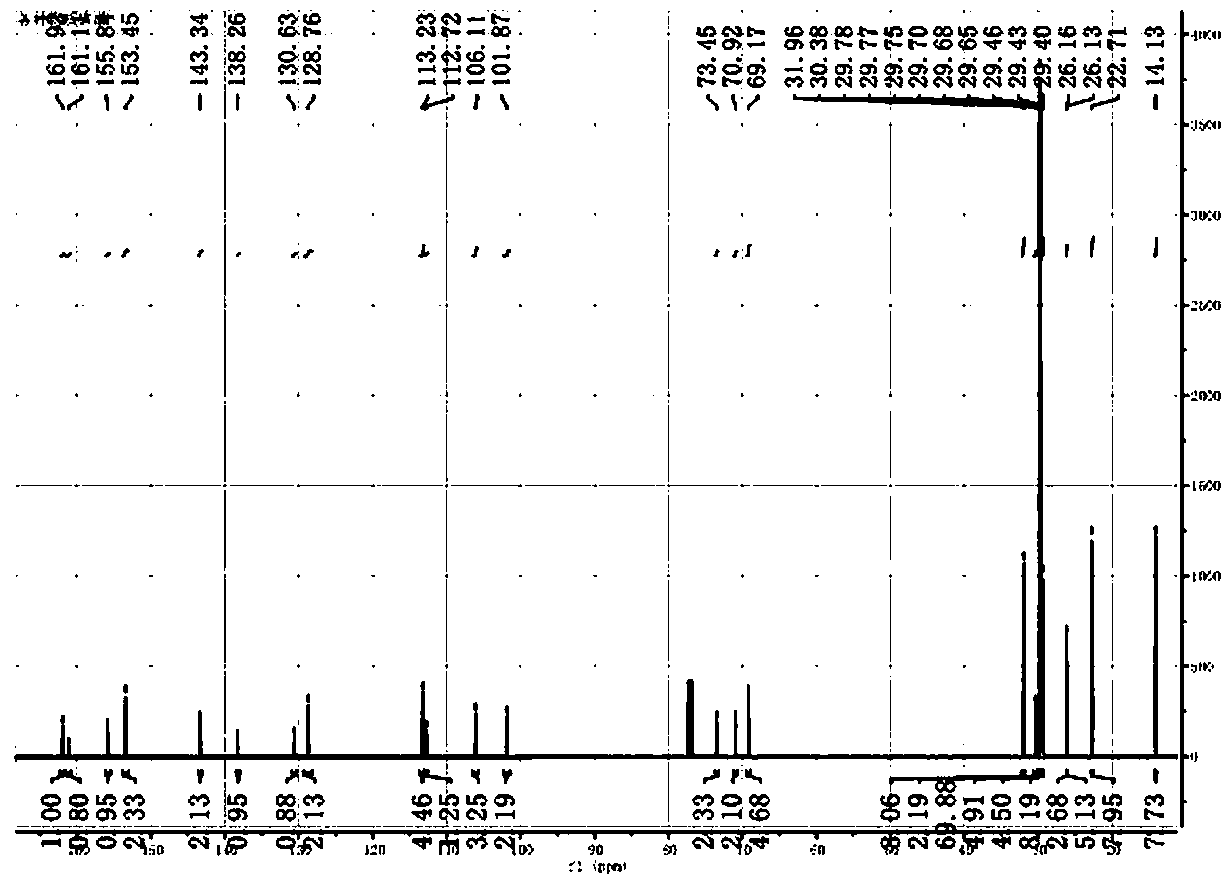
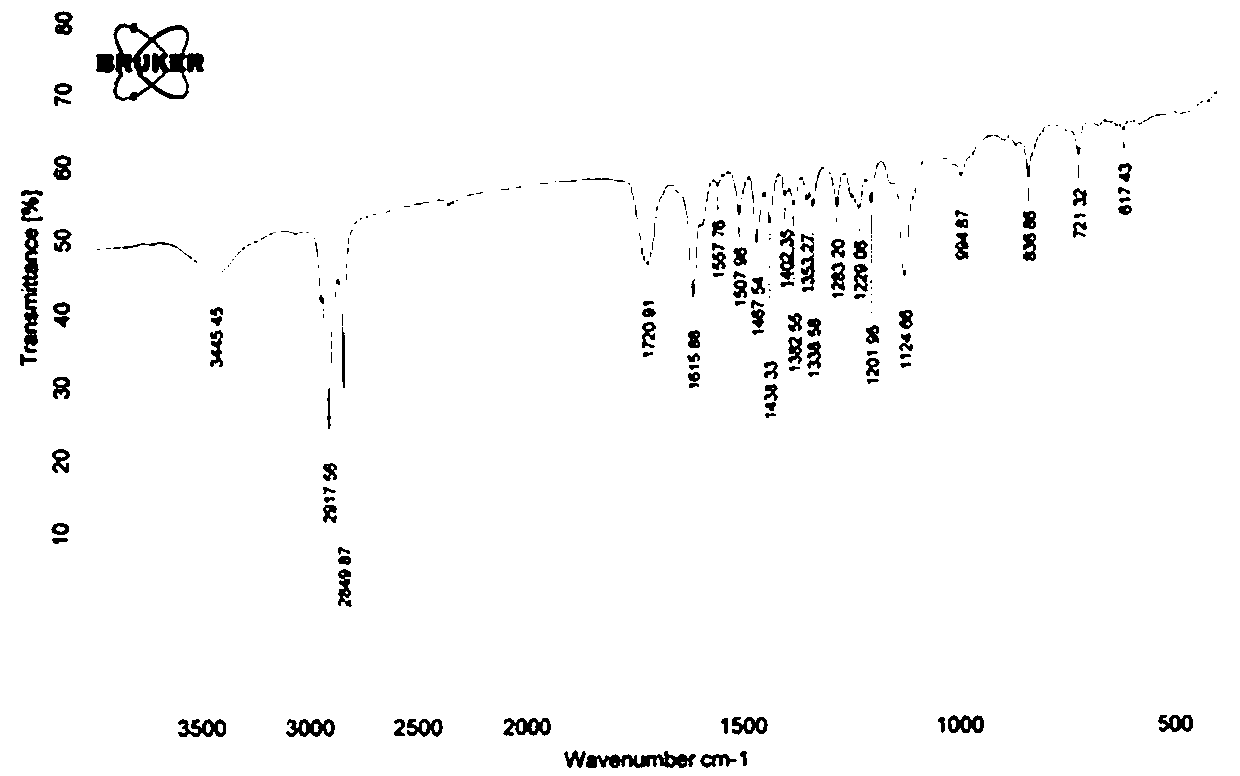
[0043] At room temperature, add 2440 mg (3 mmol) 3,4,5-hexadecyloxytoluene and 583 mg (3.6 mmol) coumarin compound 2a, 54 mg (0.1 equiv) copper acetate, 912 mg ( 2equiv) DBU, 100 in 1,4-dioxane 10mL o C under the condition of reaction for 20 hours, after the reaction is completed and cooled, add 10 mL of saturated NaCl aqueous solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, and wash with anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated, and 200-300 mesh silica gel column chromatography obtained 2720 mg of the compound 3a, with a yield of 93%. The H NMR spectrum of compound 3a is shown in figure 1 , the carbon NMR spectrum of compound 3a is shown in figure 2 , the FTIR spectrum of compound 3a is shown in image 3 .
[0044] Yellow powdery solid; mp: 69 °C.
[0045] 1 H NMR (500 MHz, CDCl 3 ): δ 7.63 (d, J = 9.5 Hz, 1 H, a), 7.37 (d, J =8.6 Hz, 1 H...
Embodiment 2
[0048] Embodiment 2: Preparation of 3b product
[0049] At room temperature, add 2440 mg (3 mmol) 3,4,5-hexadecyloxytoluene and 583 mg (3.6 mmol) coumarin compound 2b, 54 mg (0.1 equiv) copper acetate, 912 mg ( 2equiv) DBU, 100 in 1,4-dioxane 10mL o C under the condition of reaction for 20 hours, after the reaction is completed and cooled, add 10 mL of saturated NaCl aqueous solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, and wash with anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated, and 200-300 mesh silica gel column chromatography obtained 2603 mg of the compound 3b with a yield of 89%. The H NMR spectrum of compound 3b is shown in Figure 4 , the C NMR spectrum of compound 3b is shown in Figure 5 , the FTIR spectrum of compound 3b is shown in Figure 6 . 4-(3,4,5-Tris-hexadecyloxy-benzyloxy)-chromen-2-one (3b) Yellow powdery solid; mp: 77°C.
[0050] 1 H NMR (500 MHz, CDCl 3 ): δ 7.74 (d, J =...
Embodiment 3
[0053] Embodiment 3: the preparation of 3c product
[0054] In a 25 mL round bottom flask at room temperature, add 2440 mg (3 mmol) 3,4,5-hexadecyloxytoluene and 634 mg (3.6 mmol) coumarin 2c, 54 mg (0.1 equiv) copper acetate, 912 mg ( 2equiv) DBU, 100 in 1,4-dioxane 10mL o C under the condition of reaction for 20 hours, after the reaction is completed and cooled, add 10 mL of saturated NaCl aqueous solution to the system, extract 3 times with ethyl acetate, 10 mL each time, combine the organic phases, and wash with anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated, and 200-300 mesh silica gel column chromatography obtained 2668 mg of the compound 3c, with a yield of 90%. The H NMR spectrum of compound 3c is shown in Figure 7 , the C NMR spectrum of compound 3c is shown in Figure 8 , the FTIR spectrum of compound 3c is shown in Figure 9 . 4-Methyl-7-(3,4,5-tris-hexadecyloxy-benzyloxy)-chromen-2-one (3c) Yellow powdery solid; mp: 84 ℃.
[0055] 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com