Solvate crystal of PARP inhibitor and preparation method of solvate crystal
A solvate and crystal technology, applied in the field of solvate crystals of PARP inhibitors and their preparation, can solve problems such as poor fluidity and stability of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
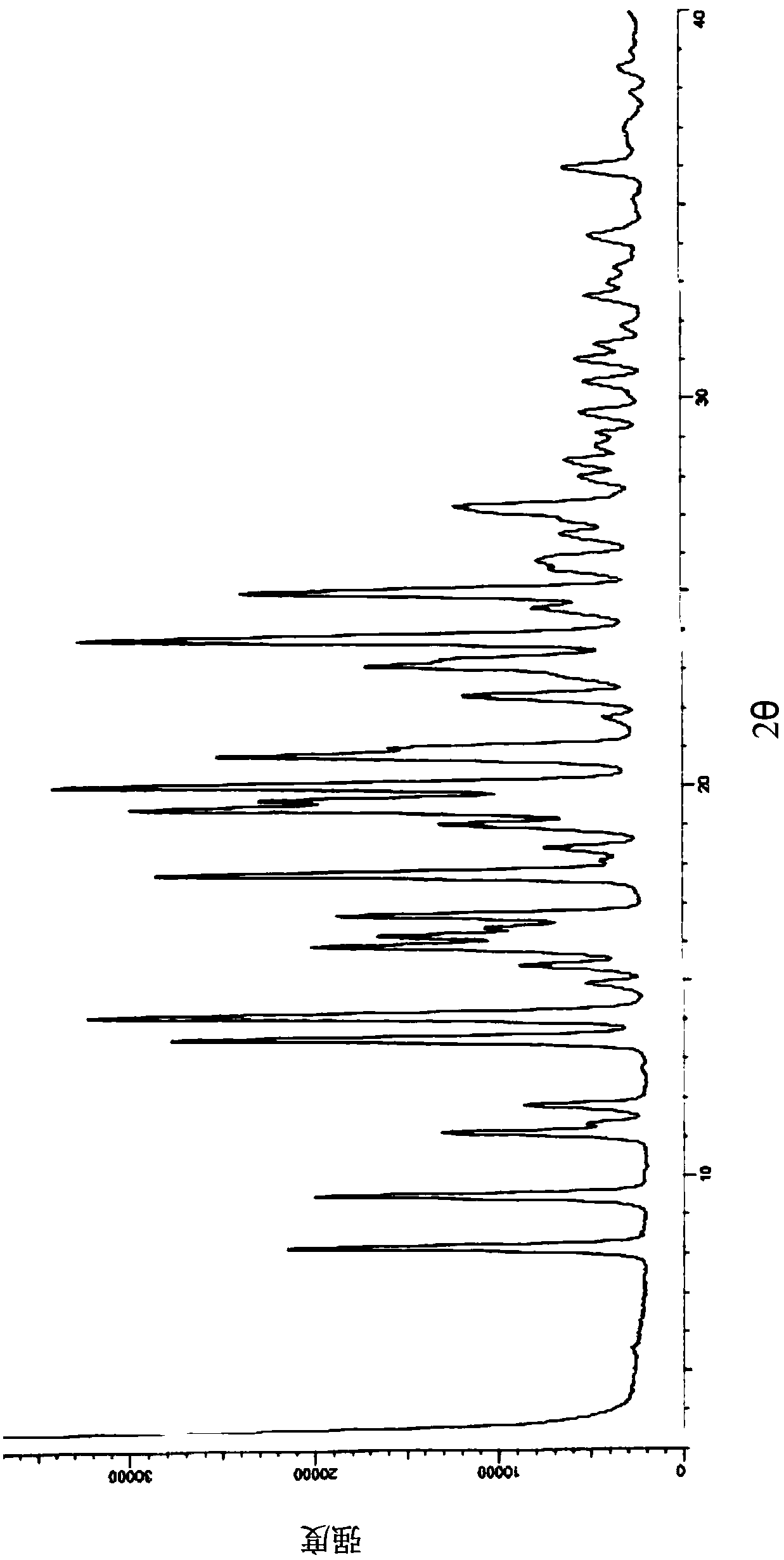
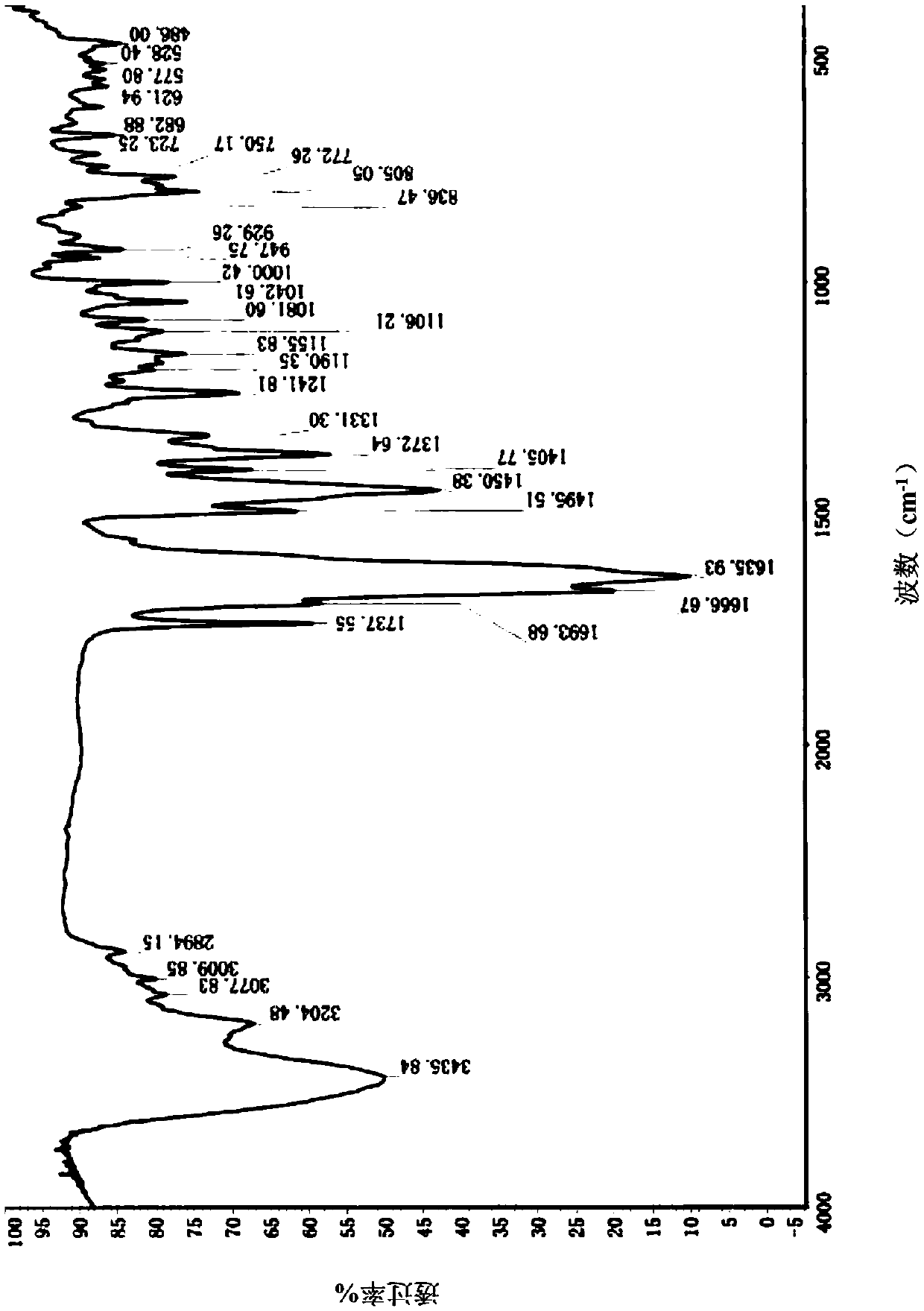
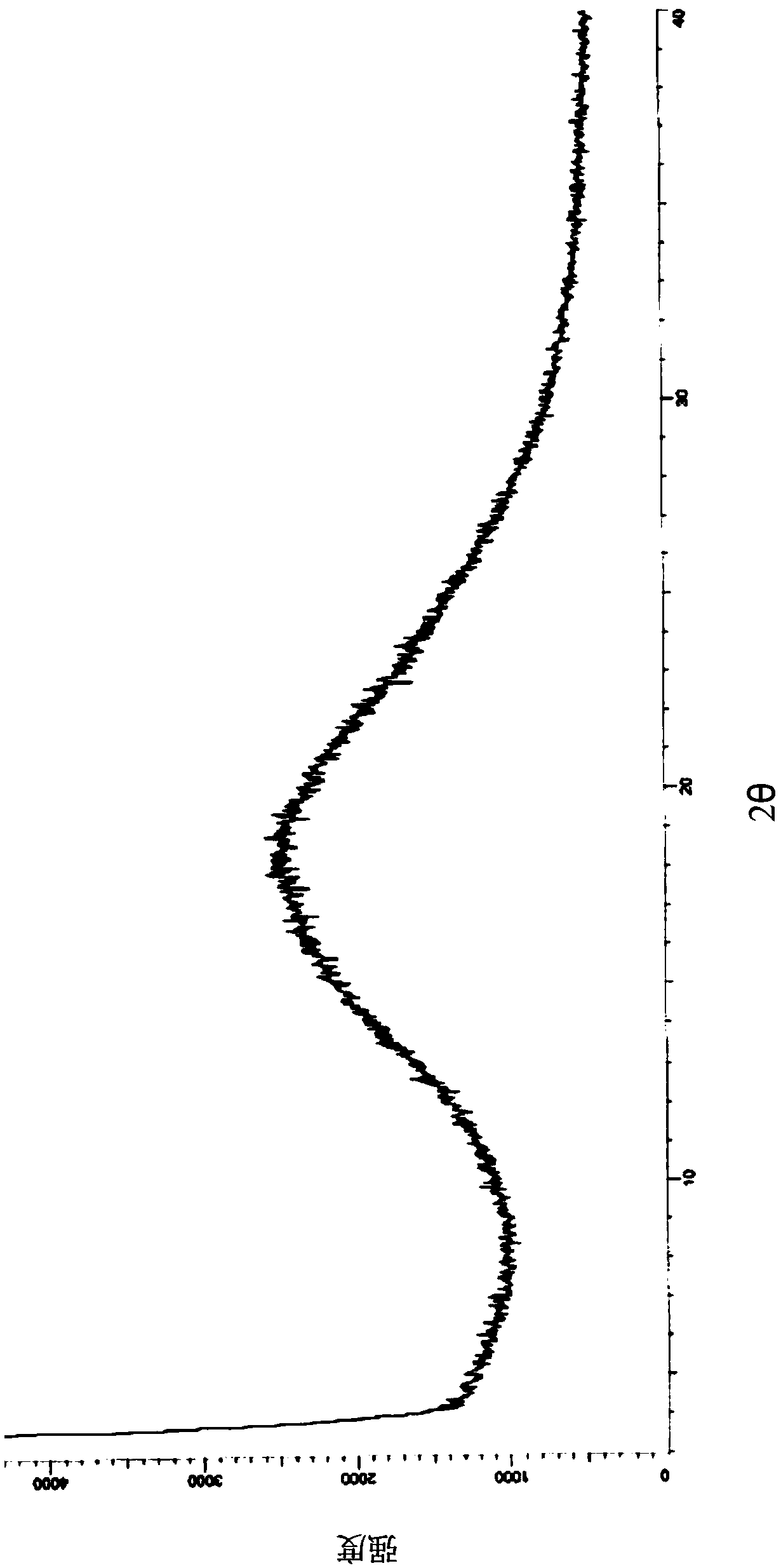
Image
Examples
Embodiment 1
[0112] 1-cyclopropyl-2-((1S,4S)-5-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl) -2,5-diazabicyclo[2,2,1]heptane-2-yl)ethane-1,2-dione (formula I) preparation
[0113] 1) 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (400mg, 1.32mmol), 2,5-diazabicyclo[2 , 2,1] Heptane-2-carboxylic acid (1S, 4S)-tert-butyl ester (665mg, 3.35mmol), 2-(7-azobenzotriazole)-N,N,N',N' -Tetramethylurea hexafluorophosphate (1.02g, 2.68mmol) and diisopropylethylamine (1.2ml, 6.7mmol) were dissolved in N,N-dimethylformamide (25ml), stirred at room temperature for 72 hours . The reaction solution was diluted with dichloromethane and water (50ml each), and the organic phase was washed with aqueous sodium bicarbonate solution and saturated brine, dried over sodium sulfate, filtered, and concentrated to give the crude product (1.23g) as a brown oil.
[0114] 2) Add trifluoroacetic acid (4 ml) to a solution of the crude product obtained in the previous step in dichloro...
Embodiment 2
[0118] Take 100g of the target product obtained in Example 1 and add 600ml of 100% (w / w) ethyl acetate to dissolve completely, and control the temperature at 10°C to stir and crystallize. Filter and rinse with an appropriate amount of ethyl acetate. The filter cake was dried under reduced pressure at 55° C. to constant weight to obtain 92.0 g of ethyl acetate solvate crystal A of formula I, with a yield of 92.0%.
Embodiment 3
[0120] Take 100 g of the target product obtained in Example 1 and add 2000 ml of ethyl acetate solution (95% (w / w) ethyl acetate, 5% (w / w) tetrahydrofuran) to dissolve completely, and control the temperature at 20° C. to stir and crystallize. Filter and rinse with an appropriate amount of ethyl acetate. The filter cake was dried under reduced pressure at 40° C. to constant weight to obtain 91.5 g of ethyl acetate solvate crystal A of formula I, with a yield of 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com