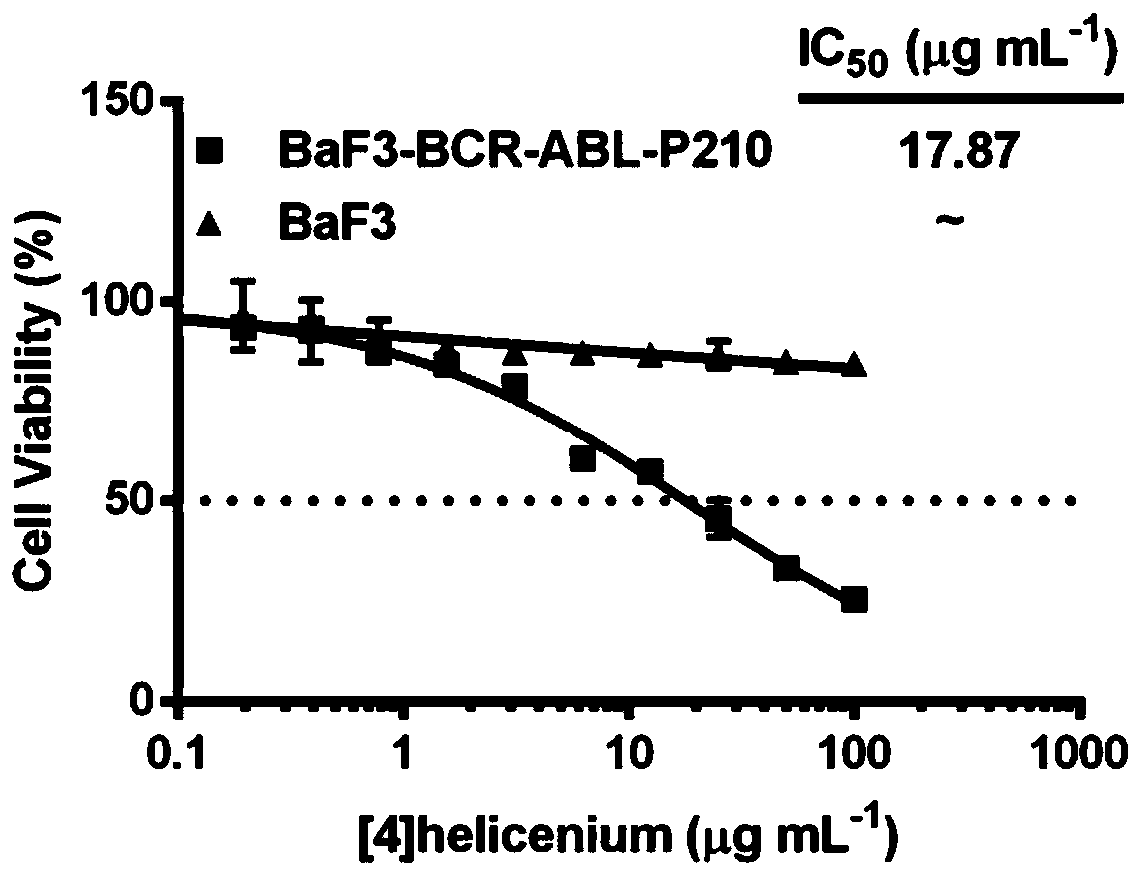
Antitumor drug, synthetic method and application
A technology of anti-tumor drugs and synthesis methods, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc. It can solve the problems of unclear biological activity, solve the problems of solubility and transportability, simple synthesis method, and low biological toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment relates to the method for preparing [4]helicenium, specifically comprising the following steps:
[0032] 4-Aza[4]helicene reference synthesis [M.J.E.Hewlins, R.Salter, Synthesis 2007,14,2164.].
[0033] 115 mg of 4-aza[4]helicene (0.5 mmol) was suspended in 3 mL of CHCl 3 and 1.5 mL of 1-bromopropane, and the mixture was stirred at 65° C. for 48 hours. As the reaction proceeded, a yellow solid was produced, and the solvent was removed by vacuum. Dissolve the solid in CH 2 Cl 2 and by adding Et 2 O precipitated and the solid was collected by centrifugation. The dissolution-precipitation-centrifugation process was repeated three times, and then the solid was kept in a vacuum oven to remove residual solvent. 123 mg of the product was obtained as a yellow solid with a yield of 70%.
[0034] Under the characterization results of the compound:
[0035] 1 H NMR (500MHz, DMSO-d6, 295K): δ10.10 (d, J = 8.6Hz, 1H), 9.61 (d, J = 5.7Hz, 1H), 8.89 (d, J = 8....
Embodiment 2
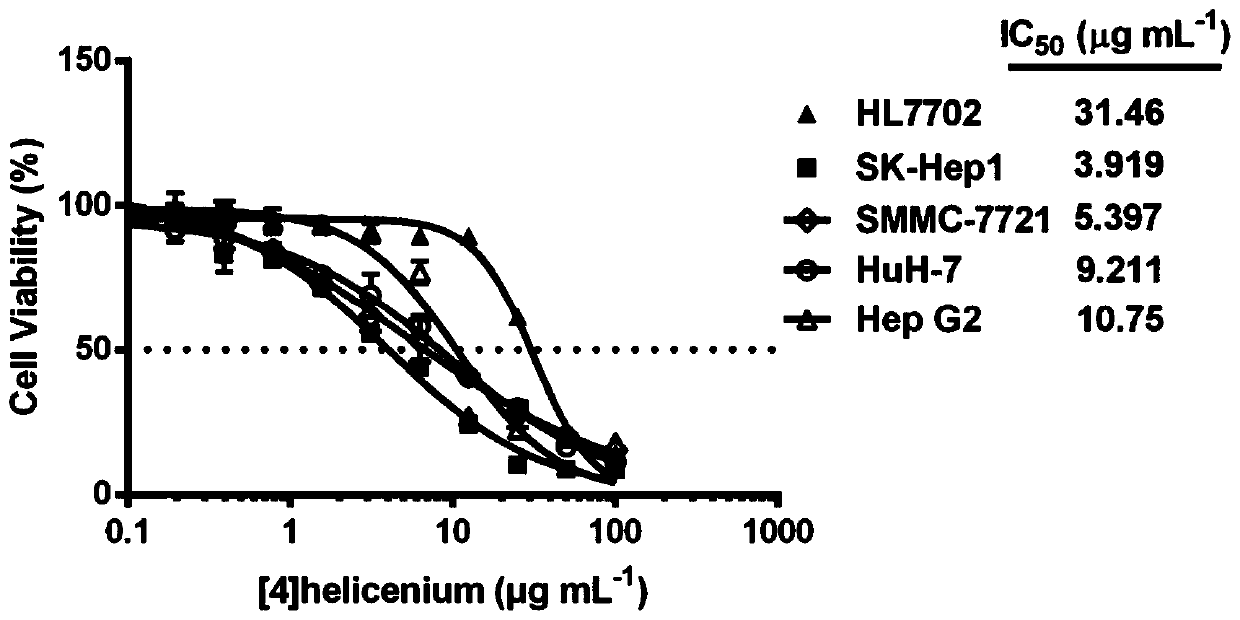
[0037] This embodiment involves detecting the selective killing effect of [4]helicenium on liver cancer cell lines, specifically including the following steps:
[0038] Hepatoma cell lines SK-Hep1, SMMC7721, HuH7, Hep G2 and liver cell line HL7702 in the logarithmic growth phase were inoculated in 96-well culture plates (1×10 4 pieces / hole). At 37°C, CO 2 Cultivate overnight in a cell incubator with a volume fraction of 5%. After the cells adhere to the wall, add 0-100 μg mL -1 The concentration of [4]helicenium was cultured for 24 hours, and 6 replicate wells were set for each concentration. After continuing to culture the cells for 24 hours, 10 μl of CCK-8 working solution was added to each well, and placed in an incubator for further incubation for 2 hours. The O.D value of each well at a wavelength of 450nm was detected on an enzyme-linked immunosorbent detector, and the survival rate of the cells was calculated. Cell survival rate (%)=(O.D value of experimental group-O...
Embodiment 3
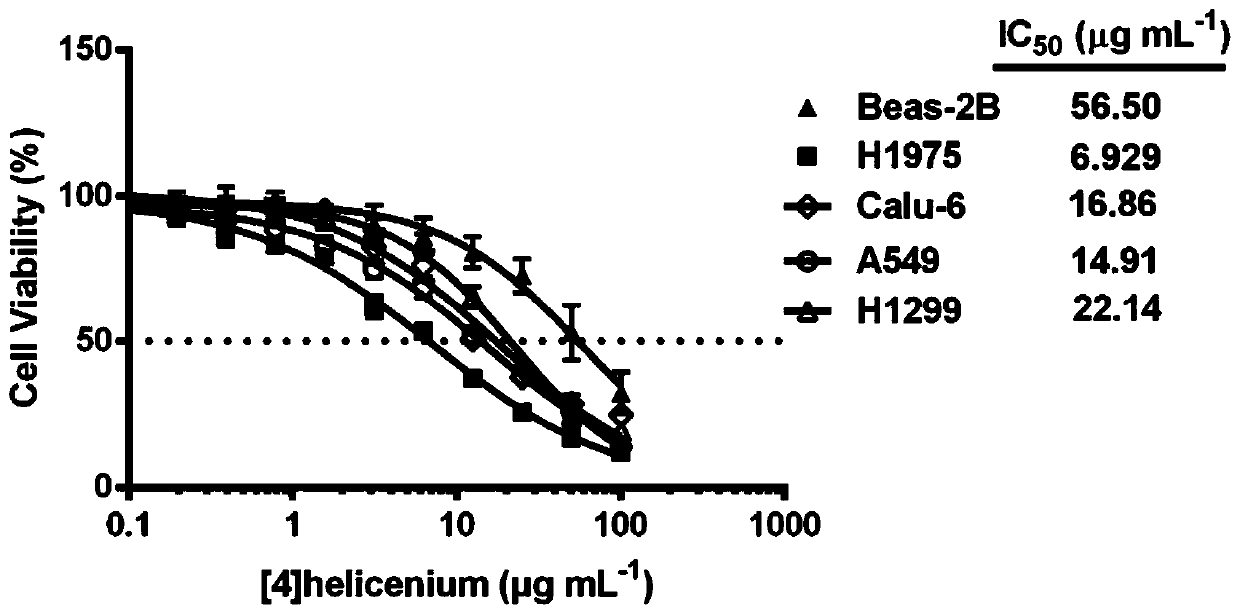
[0041] This embodiment involves detecting the selective inhibitory effect of [4]helicenium on lung cancer cell lines, specifically including the following steps:
[0042] Liver cancer cell lines H1975, Calu-6, A549, H1299 and lung cell line Beas-2B in the logarithmic growth phase were inoculated in 96-well culture plates (1×10 4 pieces / hole). At 37°C, CO 2 Cultivate overnight in a cell incubator with a volume fraction of 5%. After the cells adhere to the wall, add 0-100 μg mL -1 The concentration of [4]helicenium was cultured for 24 hours, and 6 replicate wells were set for each concentration. After continuing to culture the cells for 24 hours, 10 μl of CCK-8 working solution was added to each well, and placed in an incubator for further incubation for 2 hours. The O.D value of each well at a wavelength of 450nm was detected on an enzyme-linked immunosorbent detector, and the survival rate of the cells was calculated. Cell survival rate (%)=(O.D value of experimental group-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com