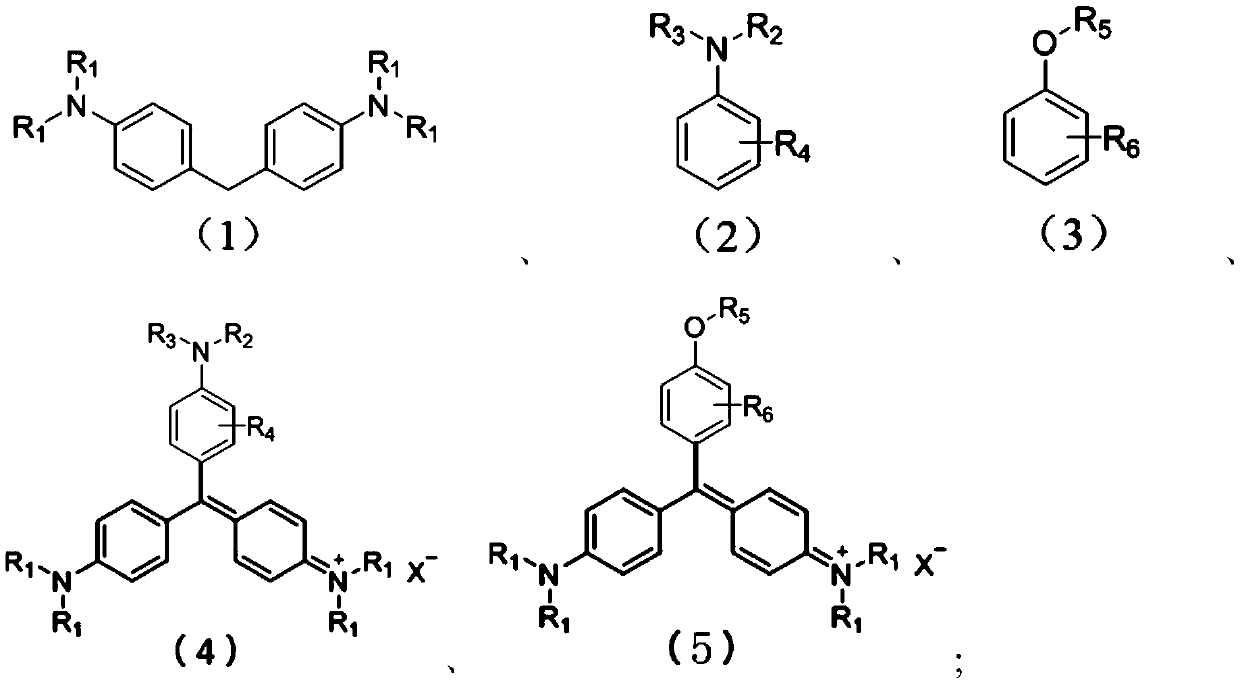
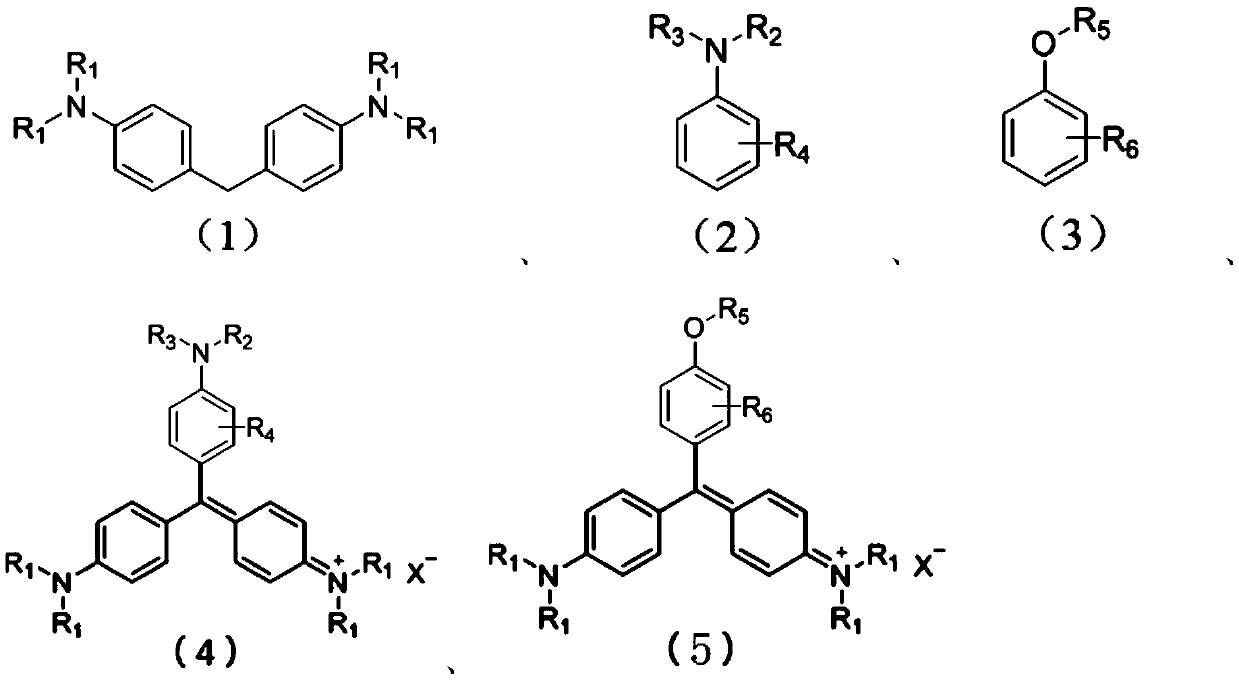
Method for preparing triarylmethane compounds and application thereof
A triaryl methane and aryl methane technology, which is applied in the preparation and application of triaryl methane compounds, can solve the problems of difficult processing, production process constraints, and high cost of raw materials, and achieve the effect of good atom economy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 250mL reaction flask equipped with a thermometer, a reflux condenser, and magnetic stirring, 26.66g (0.11mol) of N,N'-dimethylaniline, 50.87g (0.1mol) of 4,4'-bis(N, N'-Dimethyl)-diphenylmethane (methane bass), absolute ethanol 100mL, Salprn cobalt complex 1.0g, chloranil 1.5g, 5mL concentrated hydrochloric acid, heated to 65°C under stirring, passed into Oxygen, the flow rate is 15mL / min, react for 5 hours, analyze by HPLC sampling, methane bass reaction conversion is complete, stop the reaction, distill off ethanol, add 50mL water, and 10mL concentrated hydrochloric acid, heat to 75°C, filter, and cool the filtrate to Crystallize at room temperature, filter to obtain crystals with metallic luster, weigh after drying, 78.1 g, yield 95.7%. The characterization results are as follows:
[0030] Found: C, 73.70; H, 7.41; N, 10.39, Calc.for C 25 h 30 N 3 Cl: C, 73.62; H, 7.36; N, 10.31%. 1 H NMR (300MHz; CDCl 3 ;ppm) δH: 3.28(18H,s,-CH 3 ), 6.86(6H,d,J=9Hz,Ar H)...
Embodiment 2
[0032] In a 250mL reaction flask equipped with a thermometer, a reflux condenser, and magnetic stirring, 26.66g (0.11mol) of N,N'-dimethylaniline, 50.87g (0.1mol) of 4,4'-bis(N, N'-Dimethyl)-diphenylmethane (methane bass), absolute ethanol 100mL, Salen iron complex 1.0g, chloranil 1.5g, 5mL concentrated hydrochloric acid, heated to 65°C under stirring, passed into Oxygen, the flow rate is 20mL / min, react for 4 hours, sample and analyze by HPLC, methane bass reaction conversion is complete, stop the reaction, distill off ethanol, add 50mL water, and 10mL concentrated hydrochloric acid, heat to 70°C, filter, and cool the filtrate to Crystallize at room temperature, filter to obtain crystals with metallic luster, weigh after drying, 75.48g, yield 92.5%. The characterization results are as follows:
[0033] EA Found: C,73.70; H,7.41; N,10.39.Calc.for C 25 h 30 N 3 Cl: C, 73.62; H, 7.36; N, 10.31%. 1 H NMR (300MHz; CDCl 3 ;ppm) δH: 3.28(18H,s,-CH 3 ), 6.86(6H,d,J=9Hz,Ar H),7...
Embodiment 3
[0035]In a 250mL reaction flask equipped with a thermometer, reflux condenser, and magnetic stirring, put 16.42g (0.11mol) of N,N'-ethylaniline, 31.05g (0.1mol) of 4,4'-bis(N,N '-diethyl)-diphenylmethane (ethane bass), chloroform 100mL, Salen cobalt complex 0.5g, chloranil 1.0g, 10mL acetic acid, heated to 65°C with stirring, and oxygen flow rate 18mL / min, reacted for 6 hours, analyzed by HPLC sampling, the ethane bass reaction conversion was complete, stopped the reaction, distilled chloroform and acetic acid, added 50mL water, and 10mL concentrated hydrochloric acid, heated to 80 ° C, filtered, and the filtrate was cooled to Crystallize at room temperature, filter to obtain crystals with metallic luster, weigh after drying, 44.93g, yield 91.3%. The characterization results are as follows:
[0036] EA Found: C,75.57; H,8.72; N,8.61.Calc.for C 31 h 42 N 3 Cl: C, 75.66; H, 8.60; N, 8.54%; m / z (EI): 456.6 (M + -Cl - +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com