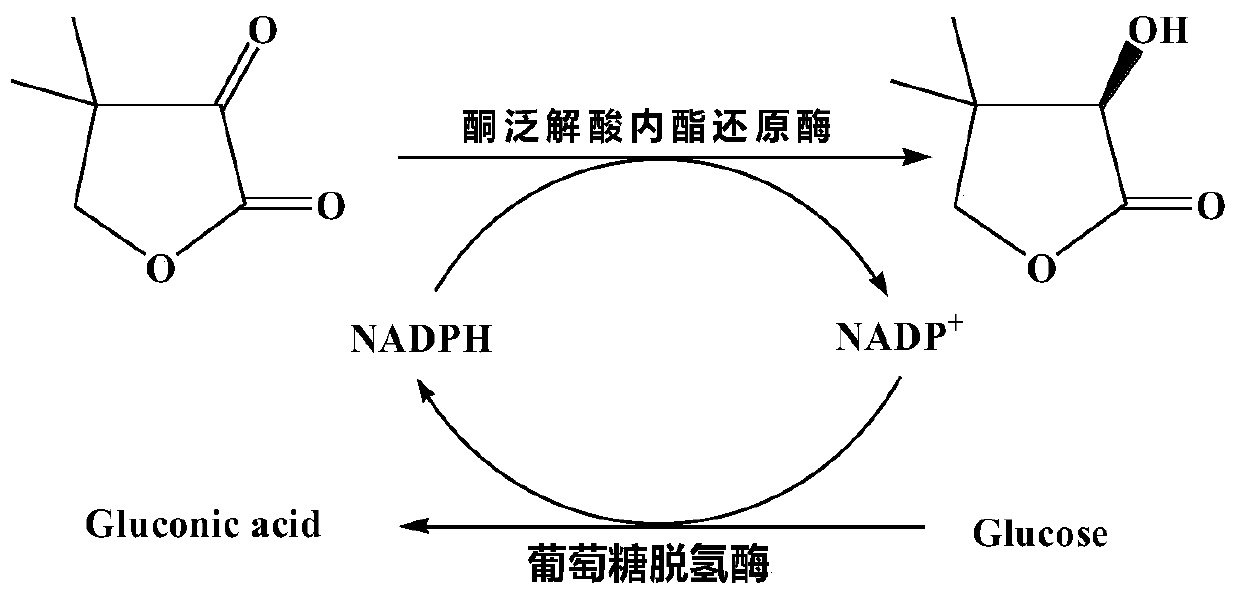
Ketopantolactone reductase and application thereof
A pantolactone and reductase technology, applied in oxidoreductase, application, genetic engineering and other directions, to achieve the effect of excellent enzyme activity, avoid additional addition, and optimize the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
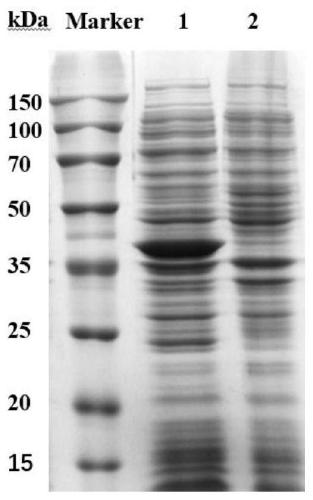
[0065] Example 1 Construction and Expression of Fission Saccharomyces Ketopantolactone Reductase Genetic Engineering Bacteria
[0066] The fission yeast ketopantolactone reductase gene KPR uses the genomic DNA of fission yeast (Schizosaccharomycespombe, from the China Industrial Microbiology Culture Collection Management Center, strain number: CICC 1056) as a template, and uses the designed primer F1( The nucleotide sequence is shown in SEQ ID NO: 6) and R1 (the nucleotide sequence is shown in SEQ ID NO: 7) for PCR amplification, and the amplification system is shown in Table 1. The PCR reaction process is as follows: 95°C pre-denaturation for 2 minutes; then, denaturation at 95°C for 20 sec, 55°C for 20 sec, 72°C for 20 sec as a cycle, and repeat this cycle 35 times; finally, 72°C for 5 min. PCR products were detected and recovered by 1% agarose gel electrophoresis. The purified PCR product was connected with the vector pEASY-Blunt E1, and the connected product was transform...
Embodiment 2
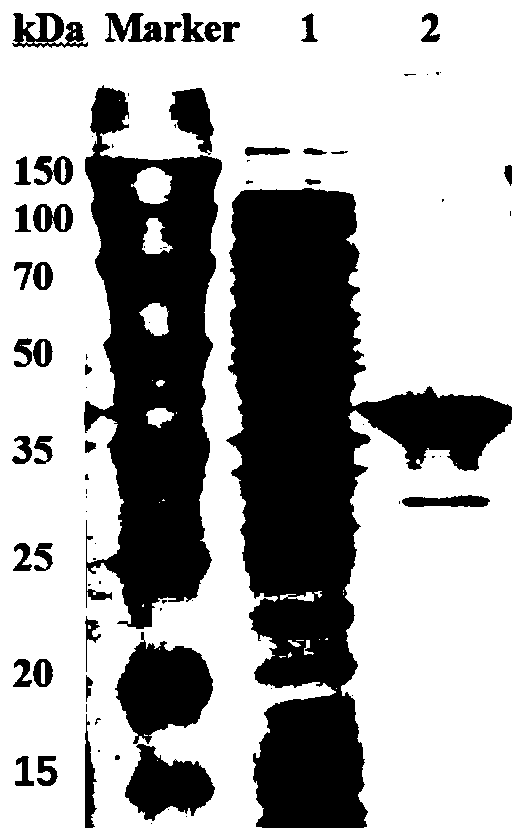
[0070] Example 2 Separation and Purification of Fission Yeast Ketopantolactone Reductase
[0071] Add appropriate amount of Tris-HCl (pH 8.0) damping solution to the wet thalline collected in Example 1 by the ratio of 1g wet thalline plus 15mL 50mM Tris-HCl buffer (pH 8.0), and ultrasonically break it for 20min at 500W (working 2s, 6s interval), the crushed solution was centrifuged at 4°C and 10000rpm for 10min, and the centrifugation was repeated three times to obtain the supernatant crude enzyme solution.
Embodiment 3
[0074] Example 3 Determination of Enzyme Activity of Fission Yeast Ketopantolactone Reductase
[0075] Enzyme activity assay method: Standard enzyme activity assay system (2.5mL) contains: 0.15mM NADPH, 0.6μg / mL fission yeast ketopantolide reductase, 10mM ketopantolide (ketopantolide 0.2MKH pH 2.0 for hydrochloride 2 PO 4 -HCl solution is prepared in the form of 1M substrate solution and added, the amount of substrate solution added is based on the amount of ketopantoolactone, and the final concentration of ketopantoolactone in the reaction system is 10mM), with 0.2M 2 HPO 4 -NaH 2 PO 4 Buffer (pH 6.0) was the reaction medium. The stated concentrations all refer to the final concentrations in the assay system. The reaction was carried out at 55°C, NADPH and the substrate were added last, and the enzyme activity was determined by detecting the change of the absorbance value of the reaction system at 340nm per minute (the molar coefficient ε of NADPH 340 =6.22mM -1 cm -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com