Protective agent, preservation method and application of low-concentration DNA reference material
A standard substance and storage method technology, applied in the direction of DNA preparation, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problem of low concentration, achieve good stability, realize the traceability of the value, and ensure the effect of accuracy and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of TP53 gene fragment
[0056] This embodiment adopts the artificial synthesis method to prepare the TP53 gene fragment standard substance, the method is as follows:
[0057] (1) Determine the TP53 gene sequence (NCBI Gene ID: 7157) according to the GeneBank database, select a 300bp gene sequence with a specific mutation as the target fragment, first synthesize a short-chain DNA containing the ctDNA sequence, and then pass PCR The short-chain DNA is connected to obtain a 300bp long-chain DNA as shown in SEQ ID NO:4;
[0058] (2) Cloning the 300bp DNA shown in SEQ ID NO:4 into the PUC57 vector to construct a plasmid DNA containing the target sequence;
[0059] (3) Using the plasmid DNA as a template, a double-stranded DNA with a length of 300 bp was amplified by PCR, which was a candidate for the standard substance of the TP53 fragment.
Embodiment 2
[0060] Example 2 Sequence verification of TP53 gene fragment
[0061] In this example, the first-generation sequencing method was used to sequence and verify the constructed plasmid DNA to identify whether the target gene fragment of TP53 had been cloned into the plasmid. Sequencing results showed that the correctness of the inserted target sequence was 100%, and the constructed plasmid could be used as a template to synthesize a double-stranded PCR product.
[0062] Subsequently, independent bidirectional sequencing was performed on the PCR amplification product to verify whether the sequence of the amplified fragment was correct. The results proved that the correctness of each amplified target sequence was 100%, completely consistent with the designed gene sequence.
Embodiment 3
[0063] Example 3 Verification of the purity of the TP53 gene fragment
[0064] This embodiment uses three methods to jointly verify the purity of the TP53 gene fragment:
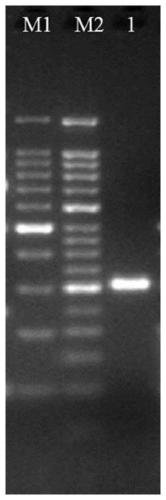
[0065] The first method uses agarose gel electrophoresis for identification, specifically: take 10 μL of TP53 gene fragment samples and load them, and use 3% agarose gel electrophoresis with a voltage of 90V and a time of 30 minutes. After the electrophoresis is completed, stain the gel. The imager analyzes the DNA bands, and judges the purity of the nucleic acid according to whether there are any bands in the picture;
[0066] The results of agarose gel electrophoresis are shown in Figure 1(A). A single DNA band can be clearly seen in lane 1 without RNA and protein contamination, which proves that the prepared TP53 gene fragment standard substance has a high purity.
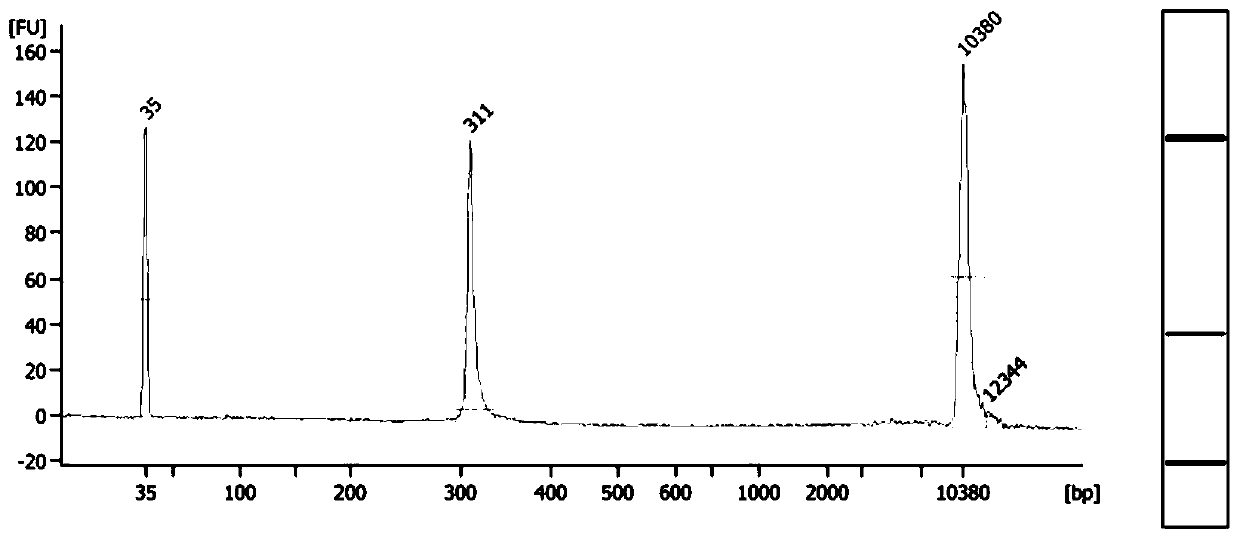
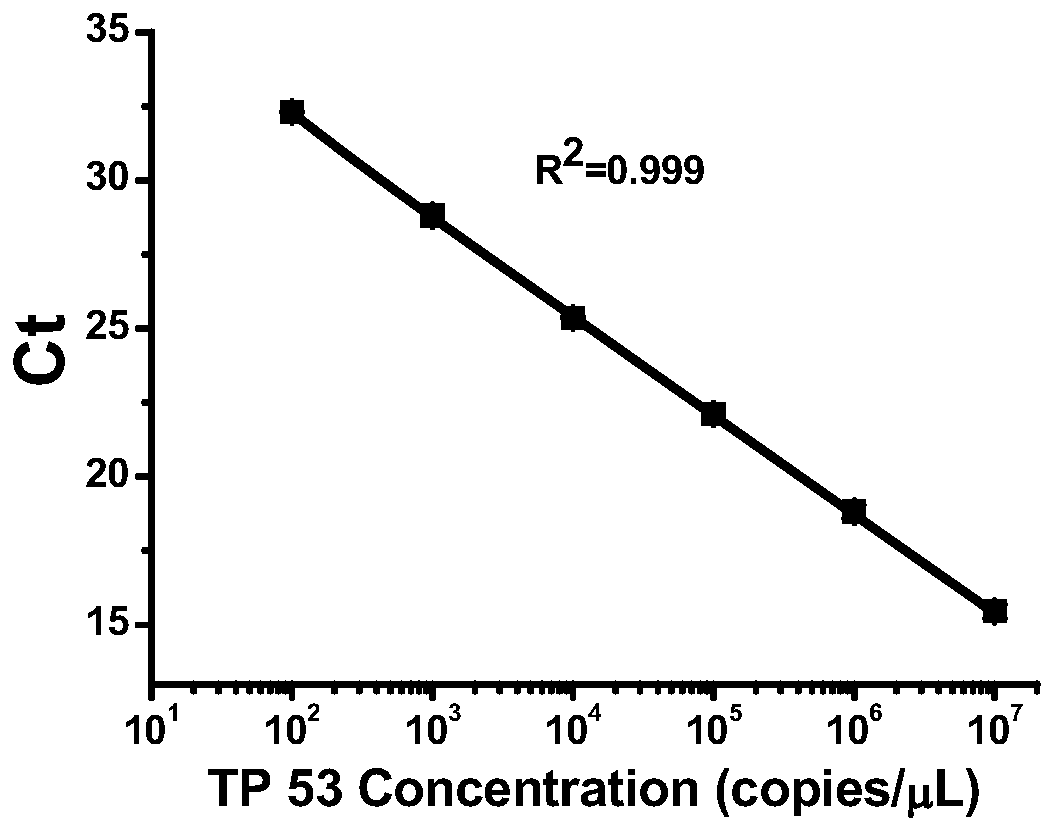
[0067] The second method uses ultraviolet spectrophotometry for identification. By detecting the ultraviolet light absorbance of the sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com