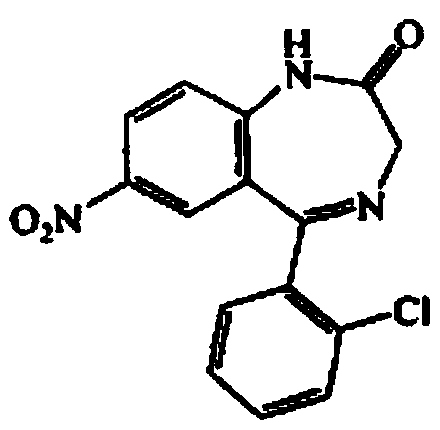
Clonazepam tablet and preparation method thereof
A technology of clonazepam tablets and nitrazepam tablets, which is applied in the field of clonazepam tablets and its preparation, can solve the problems of weak chemical stability, long disintegration time of tablets, poor formability, etc., and achieve Avoid the incidence of adverse reactions, improve the safety and effectiveness of medication, and have good molding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
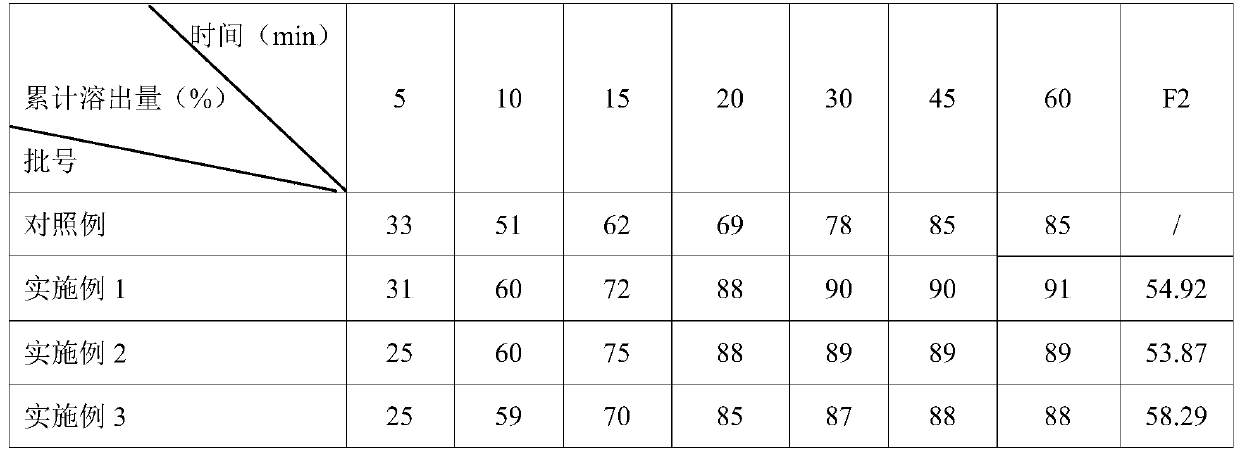
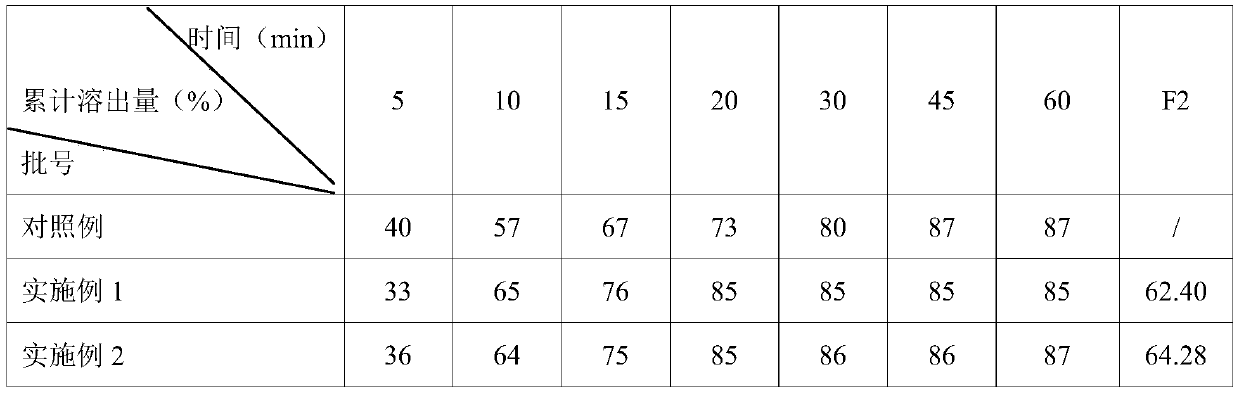
Embodiment 1
[0034] The preparation of clonazepam tablet prepares following raw material: 1.2kg clonazepam, the first filler of 30kg, the second filler of 20kg, 0.1kg binder, the first lubricant of 0.1kg, the second lubricant of 0.1kg and Appropriate amount of purified water.
[0035] Among them, the first filler is composed of lactose and microcrystalline cellulose, and the mass ratio of lactose and microcrystalline cellulose is 0.8:1.0; the second filler is composed of corn starch and pregelatinized starch, corn starch and pregelatinized starch The mass ratio of starch is 1.0:0.8; the binder is composed of corn starch and hypromellose, and the mass ratio of corn starch and hypromellose is 0.9:1.2.
[0036] This amount is used to prepare 500,000 clonazepam tablets.
[0037] The preparation method of clonazepam tablet comprises the steps:
[0038] 1) Weighing: Weigh each raw material in proportion for subsequent use;
[0039] 2) Mixing: Put the first filler and the second filler into th...
Embodiment 2
[0045] The preparation of clonazepam tablet prepares following raw material: 1.0kg clonazepam, the first filler of 25kg, the second filler of 5kg, 0.05kg binder, the first lubricant of 0.05kg, the second lubricant of 0.05kg and Appropriate amount of purified water.
[0046] Among them, the first filler is composed of lactose and microcrystalline cellulose, and the mass ratio of lactose and microcrystalline cellulose is 1:1.2; the second filler is composed of corn starch and pregelatinized starch, corn starch and pregelatinized starch The mass ratio of starch is 1.2:1.0; the binder is composed of corn starch and hypromellose, and the mass ratio of corn starch and hypromellose is 1:1.5.
[0047] This amount is used to prepare 500,000 clonazepam tablets.
[0048] The preparation method of clonazepam tablet comprises the steps:
[0049] 1) Weighing: Weigh each raw material in proportion for subsequent use;
[0050] 2) Mixing: Put the first filler and the second filler into the ...
Embodiment 3
[0056] The preparation of clonazepam tablet prepares following raw material: 1.5kg clonazepam, the first filler of 50kg, the second filler of 50kg, 1.5kg binder, the first lubricant of 1kg, the second lubricant of 0.5kg and purified Appropriate amount of water.
[0057] Among them, the first filler is composed of lactose and microcrystalline cellulose, and the mass ratio of lactose and microcrystalline cellulose is 0.5:0.8; the second filler is composed of corn starch and pregelatinized starch, corn starch and pregelatinized starch The mass ratio of starch is 0.8:0.6; the binder is composed of corn starch and hypromellose, and the mass ratio of corn starch and hypromellose is 0.8:1.0.
[0058] This amount is used to prepare 500,000 clonazepam tablets.
[0059] The preparation method of clonazepam tablet comprises the steps:
[0060] 1) Weighing: Weigh each raw material in proportion for subsequent use;
[0061] 2) Mixing: Put the first filler and the second filler into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com