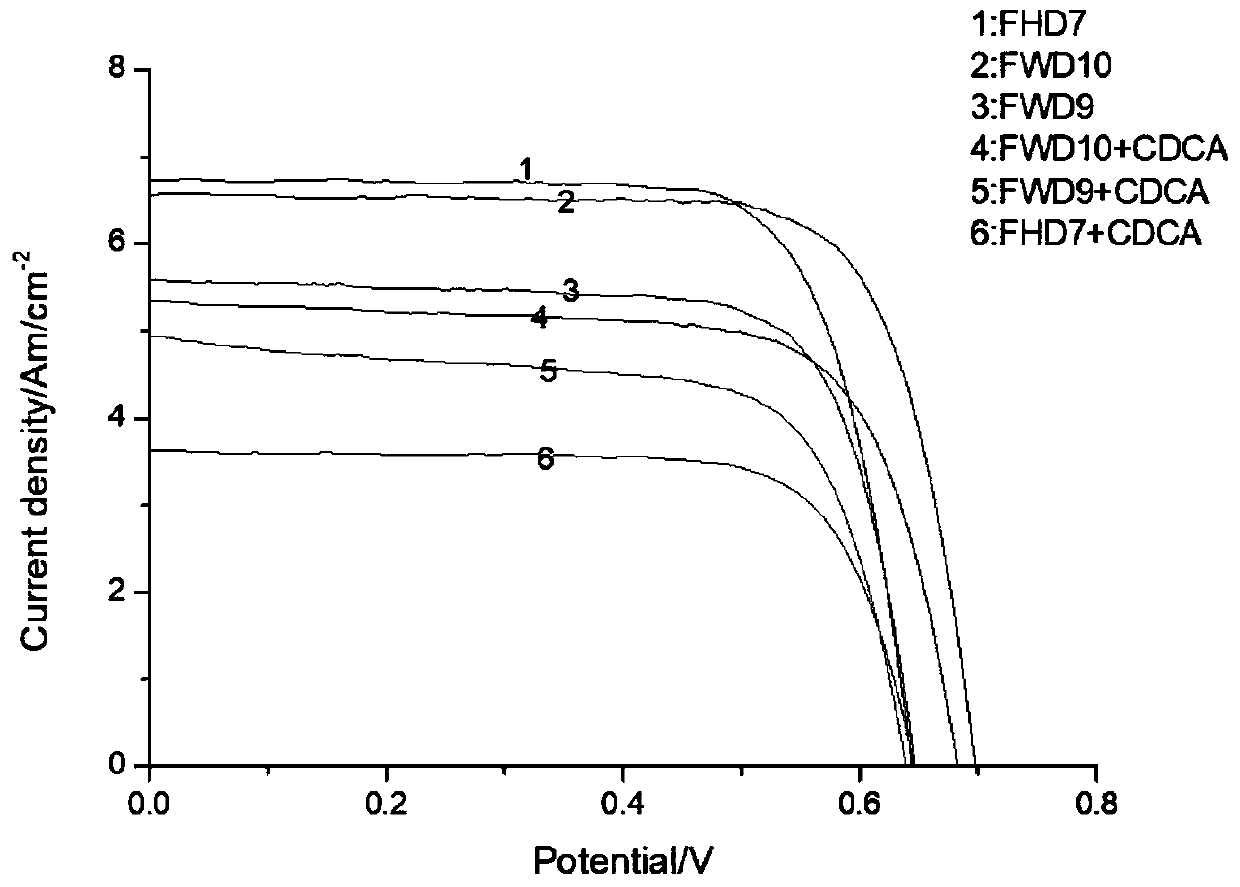
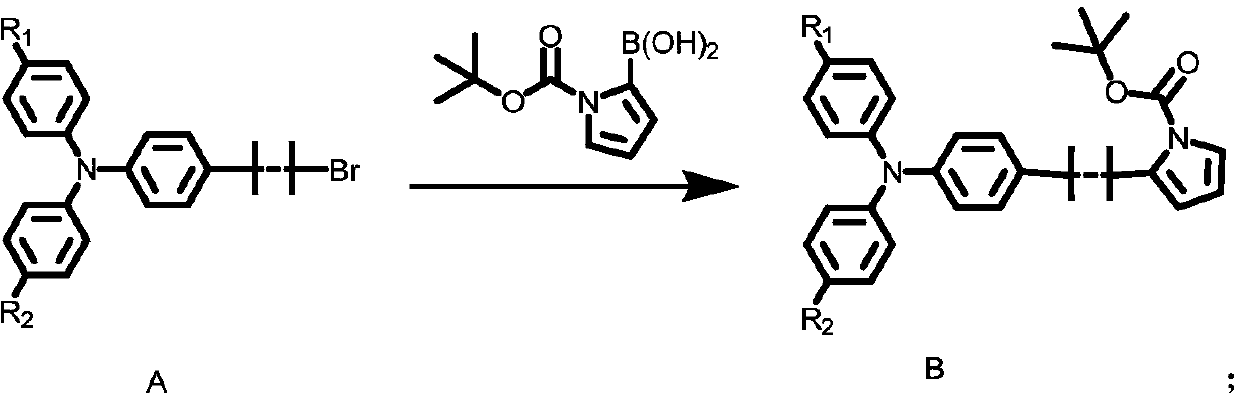
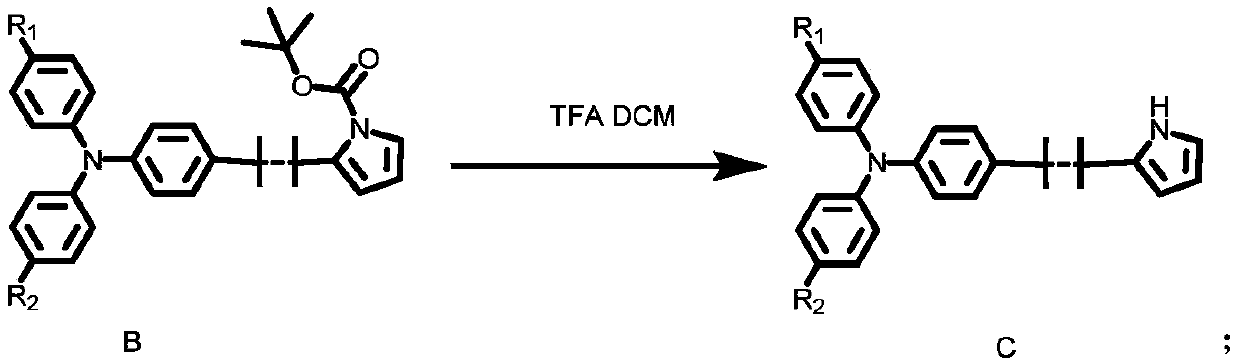
Triphenylamine organic dye and synthesis method thereof
An organic dye and synthesis method technology, applied in the field of dye-sensitized solar cell sensitizers, can solve problems such as low photoelectric conversion efficiency, and achieve the effects of favorable electron transport, good application prospects and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Dye FWD 9:
[0047] Synthesis of Compound 1: Dissolve 4-bromotriphenylamine (1.3030g, 4mmol) in anhydrous N,N-dimethylformamide (DMF) (9.3mL) under nitrogen atmosphere, and stir at 0°C for 15min , to this solution was added dropwise POCl 3 (8mL, 100mmol), the solution gradually turned blue-yellow and solid was produced, and continued to stir at 0°C for 15min, and then reacted the mixture at 100°C for 20h. After the reaction is complete, add water dropwise to the reaction system at 0°C, filter to obtain the filter residue, and filter the residue with CH 2 Cl 2 Extraction, the organic layer was washed with saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4Drying, rotary evaporation, PE / DCM as eluent, and column chromatography gave Intermediate 1 (light yellow crystal, 1.1353g), with a yield of 74.6%.
[0048] Synthesis of Compound 2: Under a nitrogen atmosphere, Intermediate 1 (1.2383g, 3.3mmol) and diethyl benzylphosphonate (1.5483g, 9.9...
Embodiment 2
[0061] Synthesis of Dye FWD10:
[0062] Synthesis of compound 7: 4-bromoaniline (1.4516g, 4.5mmol), 1-Boc-pyrrole-2-boronic acid (863.3mg, 4.1mmol), tetrakis(triphenylphosphine) palladium were added successively in a 250mL two-necked flask (284.3mg, 6%mmol), sodium carbonate (1.3037g, 13.5mmol), under the condition of nitrogen protection, solvent tetrahydrofuran (52mL) and water (13mL) were added respectively, and the reaction system was refluxed overnight. After the reaction is complete, add saturated NH 4 Cl solution with CH 2 Cl 2 Extraction, organic phase with anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, PE / DCM as eluent, column chromatography gave Intermediate 7 (white solid, 1.3061g), yield 70.7%
[0063] 1 H NMR (500MHz, CDCl 3 )δ (ppm): 7.34 (dd, J = 3.2, 1.8Hz, 1H), 7.25 (t, J = 7.6Hz, 4H), 7.20 (d, J = 8.5Hz, 2H), 7.11 (d, J = 7.7Hz, 4H), 7.04(d, J=8.5Hz, 2H), 7.01(t, J=7.3Hz, 2H), 6.21(t, J=3.3Hz, 1H), 6.17(dd, J=3.1, 1.8Hz,1H),1.41(s,9H).
...
Embodiment 3
[0073] Synthesis of Dye FHD 7:
[0074] Synthesis of compound 12: Under nitrogen atmosphere, intermediate 11 (1.3698g, 4mmol) and phenylselenite anhydride (1.7286g, 4.8mmol) were dissolved in chlorobenzene (30mL), and the reaction was refluxed for 12h. After the reaction is complete, add saturated NH 4 Cl solution with CH 2 Cl 2 Extraction, organic phase with anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, PE / DCM as eluent, and column chromatography gave intermediate 13 (white solid, 1.1970 g) with a yield of 80.4%.
[0075] 1 H NMR (300MHz, CDCl 3 )δ (ppm): 7.83 (d, J = 7.2Hz, 4H), 7.42-7.31 (m, 4H), 7.26-7.16 (m, 4H), 7.04 (d, J = 7.5Hz, 2H), 6.59 ( d,J=8.1Hz,2H).
[0076] Synthesis of Compound 13: Intermediate 12 (745.9mg, 2.0mmol), 3,6-dibromo-1,2-phenylenediamine (638.2mg, 2.4mmol) and p-toluenesulfonic acid (29.5mg, 8mol%) Add it to a 100 mL two-neck flask, add chloroform (20 mL) under a nitrogen atmosphere, and reflux the reaction overnight. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com