Method for preparing 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-formic acid
A technology of hydroxybenzene and tetrahydrofuran, applied in the field of organic compound preparation, can solve problems such as lack of mature technology, and achieve the effects of improving economic benefits, cheap and easy-to-obtain raw materials, and reaction cost advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
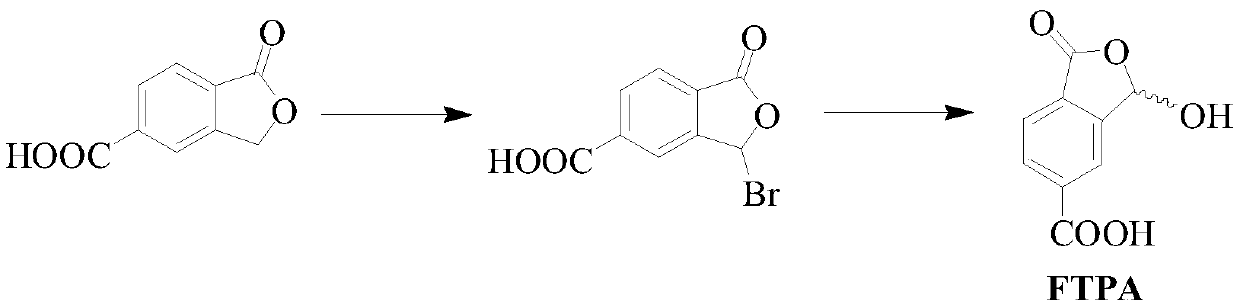
[0032] The preparation method of 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-carboxylic acid The synthetic route is as follows:
[0033]
[0034] Proceed as follows:
[0035] first step reaction
[0036] Add 4.4g of magnesium powder, elemental iodine and magnetons into a 500mL three-necked flask, add a reflux tube to the middle mouth of the three-necked flask, and use a tee to connect the nitrogen balloon on the reflux tube; install a constant pressure dropping funnel on the left side of the three-necked flask , the constant pressure dropping funnel was equipped with 9.075g of 2,5-dibromotoluene in tetrahydrofuran solution; the right side of the three-necked flask was sealed with a reverse rubber stopper, and the nitrogen was replaced three times; Tetrahydrofuran solvent 150mL, the reaction solution was heated to 50°C, and the tetrahydrofuran solution containing 2,5-dibromotoluene was slowly added dropwise to the reaction solution, and after the dropwise addition, the reaction...
Embodiment 2
[0040] first step reaction
[0041] Add 5.3g of magnesium chips, iodine element and magnetons into a 500mL three-necked flask. A reflux tube is added to the mouth of the three-necked flask, and a drying tube is connected to the reflux tube. A constant-pressure dropping funnel is installed on the left side of the three-necked flask. The dropping funnel is filled with 5.847g of 2,5-dichlorotoluene in tetrahydrofuran solution; add 150mL of freshly treated tetrahydrofuran solvent from the right bottle mouth, heat the reaction solution to 65°C, and dissolve the solution containing 2,5-dichlorotoluene The tetrahydrofuran solution was slowly added dropwise to the reaction solution, and after the dropwise addition, continued to react at 65°C for 8 hours; slowly bubbled carbon dioxide gas into the reaction solution, and at the same time opened the tee to connect to the atmosphere, and stopped blowing after 1 hour of reaction; wait for the reaction solution to cool to room temperature, ...
Embodiment 3
[0044] The difference between the first step reaction and the first step reaction in Example 1 is that 12.4881 g of 2,5-diiodotoluene is used instead of 2,5-dibromotoluene; the reaction is carried out under the protection of argon, and the reaction is carried out at 65°C After 2 hours, 4.2 g of yellow solid was obtained, and the calculated yield was 60%.
[0045] The difference between the second step reaction and the second step reaction of Example 1 is that the chromium trioxide is 8.0g, the reaction time is extended to 12h at 25°C, the extraction solvent is dichloromethane, and the desiccant is anhydrous Magnesium sulfate, 1.5 g of the target compound was obtained, and the calculated yield was 39%.
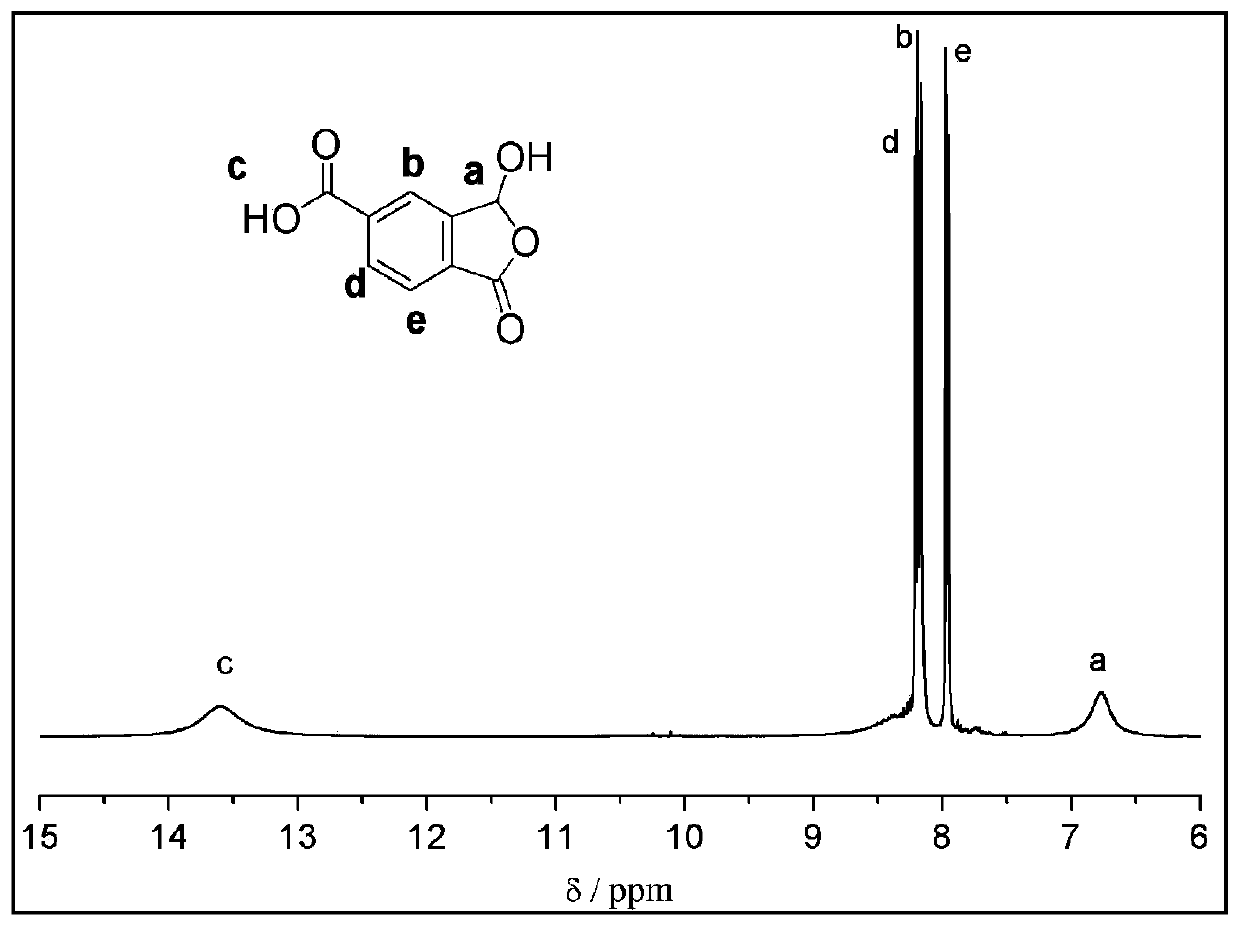
[0046] The proton NMR spectrum of the product is as follows:
[0047] 1 HNMR (400MHz, DMSO-d 6 )δ13.61(br,1H),8.50-8.0(br,1H),8.20(d,J=8.0Hz,1H),8.17(s,1H),7.95(d,J=8.0Hz,1H), 6.77(s,1H), the spectrum is as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com