Method for solid-phase macrosynthesis of non-noble metal oxygen reduction catalyst, and catalyst and application thereof
A non-precious metal and catalyst technology, which is applied in the field of solid-phase macro-synthesis of non-precious metal oxygen reduction catalysts, can solve the problems of difficult control of preparation process conditions, complicated preparation methods, unfavorable industrial production of catalysts, etc. Simple, convenient and easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Mix 0.600g nitrilotriacetic acid with 0.77g cobalt chloride (the molar ratio of nitrilotriacetic acid to cobalt chloride is 1:2), and solid-phase grind fully, place in the quartz tube of tube furnace, use Remove the air with nitrogen for half an hour, then raise the temperature to 800°C at 3°C / min, and heat-treat for 2 hours under the protection of nitrogen to obtain the heat-treated product; collect the heat-treated product, wash with water, centrifuge and vacuum-dry at 60°C, A non-noble metal oxygen reduction catalyst is obtained.
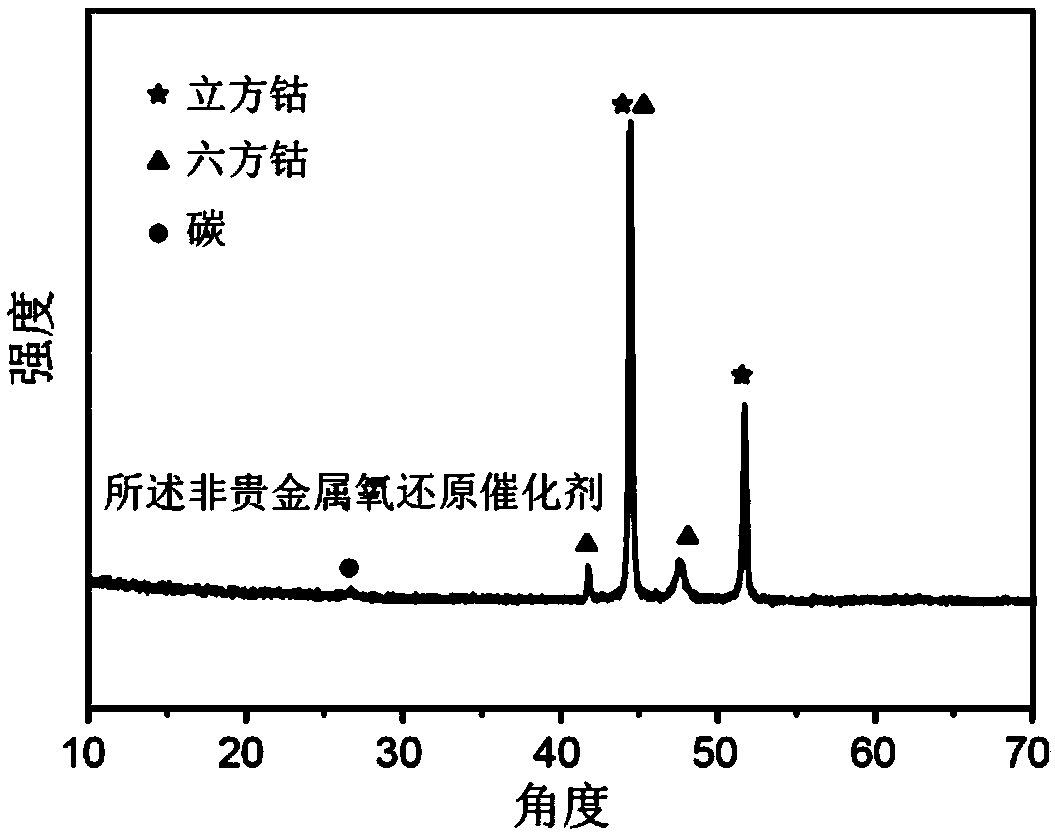
[0035] The X-ray powder diffraction curve of the non-noble metal oxygen reduction catalyst prepared in this embodiment is as follows figure 1 Shown, as can be seen from the figure, the catalyst prepared in the present embodiment contains metallic cobalt and graphitized carbon.
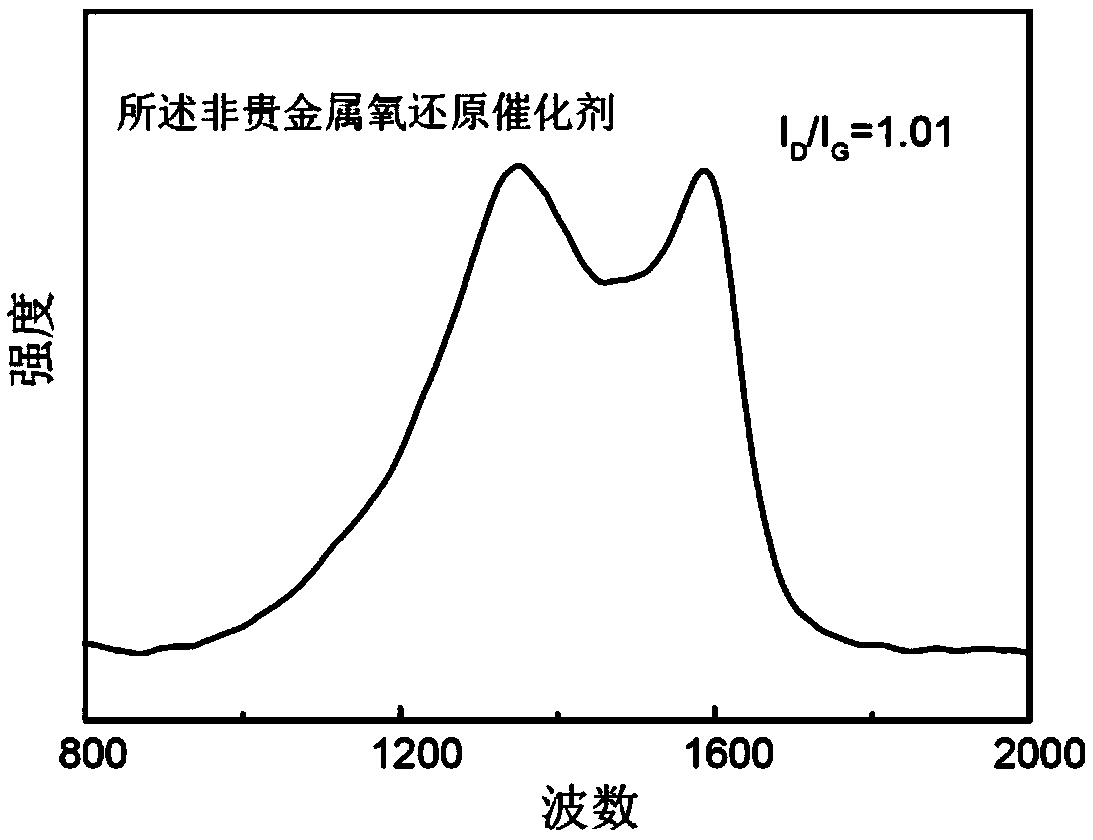
[0036] The laser Raman spectrum curve of the non-noble metal oxygen reduction catalyst prepared in this embodiment is as follows figure 2 As shown, it can be see...
Embodiment 2
[0049] Other preparation processes are all the same as in Example 1, only the chelating agent is changed into ethylenediaminetetraacetic acid, and the consumption is 0.92g (the molar ratio of ethylenediaminetetraacetic acid to cobalt chloride is 1:2).
[0050] The resulting non-precious metal oxygen reduction catalyst has a half-wave potential of about 0.80 volts (relative to the standard hydrogen electrode) obtained by testing the oxygen reduction curve in a 0.1 mole per liter potassium hydroxide solution, which is only higher than the half-wave potential of the commercial platinum-carbon catalyst. The potential is 0.85 volts lower than 50 millivolts, and the number of electron transfers corresponding to 0.3V-0.6V obtained from the K-L equation is also close to 4, so it shows good oxygen reduction catalytic performance. In the stability test of 20000s, the current drops to 80% of the original, and it also has methanol resistance. But there is no coral-like catalyst morphology...
Embodiment 3
[0052] Other preparation processes are all the same as in Example 1, only the chelating agent is changed into ethylenediaminetetraacetic acid, and the consumption is 1.07g (the mol ratio of ethylenediaminetetraacetic acid and cobalt chloride is 1:2).
[0053] The resulting non-precious metal oxygen reduction catalyst has a half-wave potential of 0.81 volts (relative to the standard hydrogen electrode) obtained by testing the oxygen reduction curve in a 0.1 mole per liter potassium hydroxide solution, which is only higher than the half-wave potential of the commercial platinum carbon catalyst. 0.85 volts is 40 millivolts lower, and the number of electron transfers corresponding to 0.3V-0.6V obtained from the K-L equation is also close to 4, so it shows good oxygen reduction catalytic performance. In the stability test of 20000s, the current drops to 82% of the original value, and it also has the ability to resist methanol. But it does not possess the coral-like catalyst morphol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| mesopore | aaaaa | aaaaa |
| micropore | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com