A kind of degradable high pH sensitive polymer and its preparation method and application
A polymer and sensitive technology, applied in the field of drug carriers, can solve the problems of difficult adjustment of biodegradation performance at mutation points, wide mutation range, and low drug loading capacity, and achieve good blood compatibility, low surface energy, and good encapsulation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
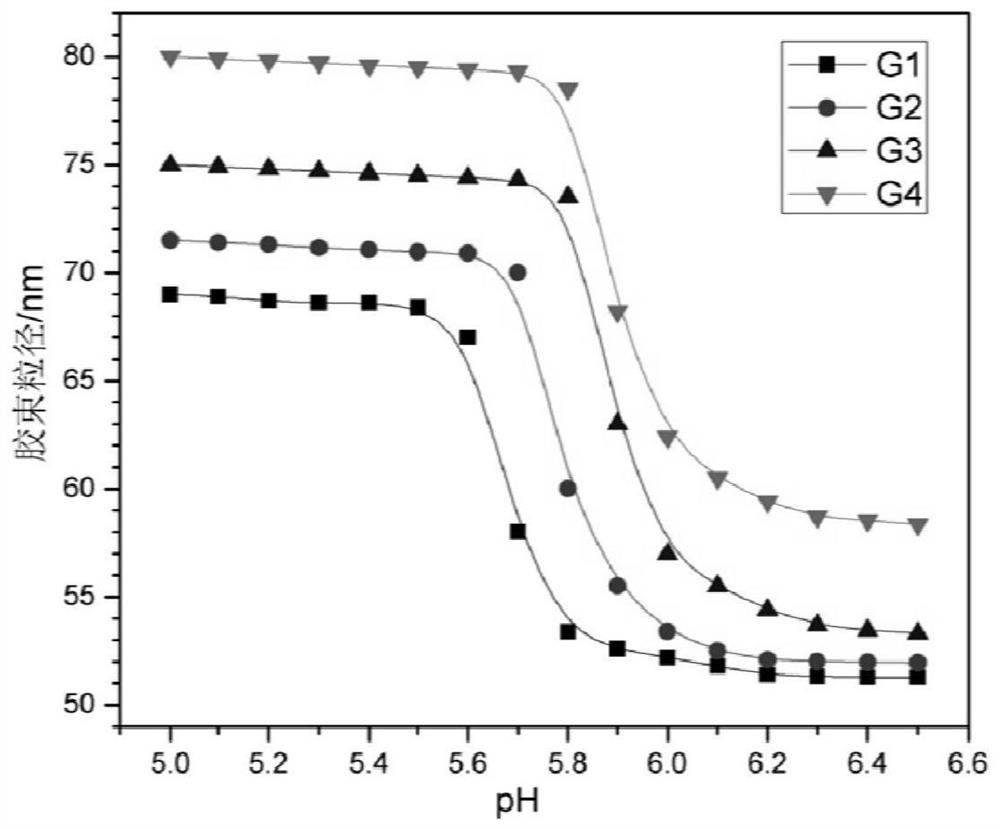
Examples
Embodiment 1
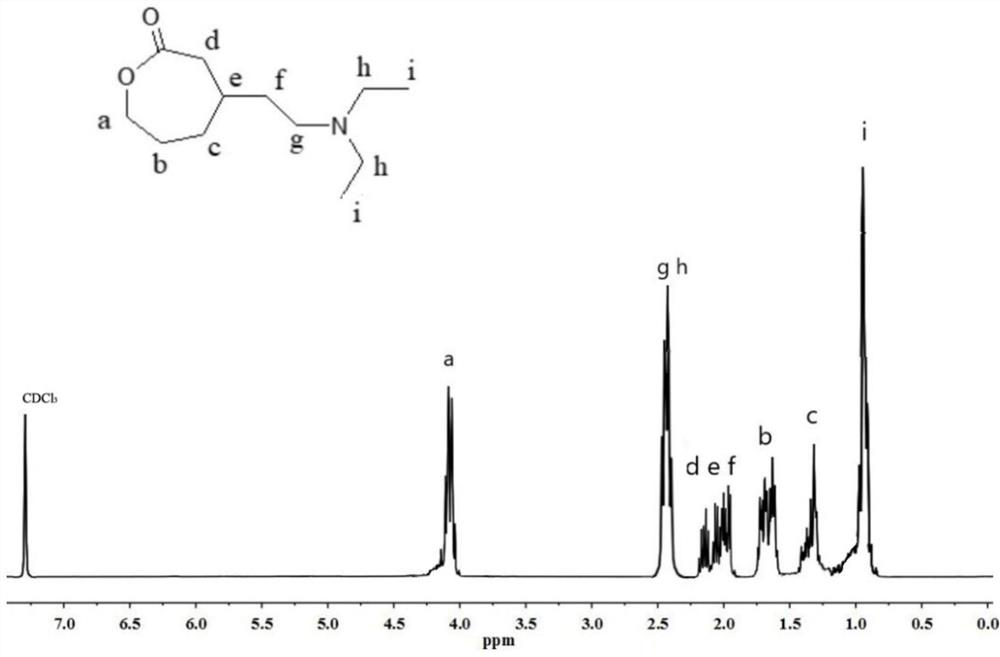
[0058] Add 0.5g benzoyl peroxide (Bz) to 11mL 6-caprolactone (I) 2 o 2 ), and 20mL of carbon tetrachloride solution of bromine (solution concentration is 0.8g / mL) was added dropwise, and reacted at room temperature for 1h. Dry over magnesium sulfate and filter to obtain the carbon tetrachloride solution of II; add 15 g of anhydrous sodium carbonate to the filtrate, heat to 70 ° C for 1 hour, and filter to obtain the carbon tetrachloride solution of III; Slowly add it dropwise to 20 mL of triethylaminomagnesium bromide in tetrahydrofuran (concentration: 1 g / mL) containing 0.4 g CuCl, and react at -20°C for 2 h. After the reaction was completed, the temperature of the reaction solution was raised to -5°C, and an equal volume of saturated ammonium chloride solution was slowly added dropwise to the reaction solution and continued to stir for 20 min, filtered by suction, separated, and the organic phase was washed 3 times with a saturated ammonium chloride solution. It was dried ...
Embodiment 2
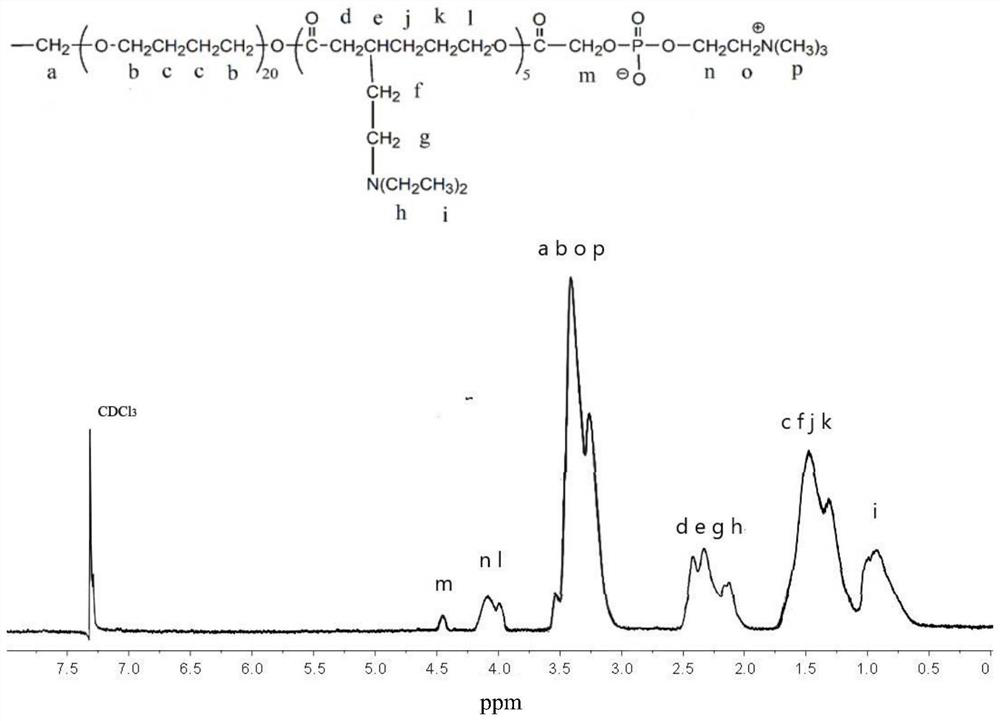
[0061] Preparation of prepolymer A: four-arm polytetrahydrofuran polyol (7.5g, M n =1500g / mol), 17.12g of 3-(triethylamino)-6-caprolactone and 25mg of stannous octoate were dissolved in 20mL of N,N-dimethylformamide, and argon gas was introduced for 5 minutes to remove oxygen. The temperature of the oil bath was raised to 70°C, the reaction time was 48h, and then lowered to room temperature, settled three times with 120mL of methanol, filtered with suction, and vacuum-dried at 40°C to constant weight to obtain prepolymer A.
[0062] Preparation of the polymer shown in formula I: 9.80g of prepolymer A, 2.02g of carboxymethoxyphosphorylcholine and 0.98g of dimethylaminopyridine were dissolved in 20mL of dichloromethane and 1.65g of N,N'-bicyclic Hexyl carboximide was protected by dry argon, and the reaction was carried out under magnetic stirring at room temperature for 24 hours. Suction filtration, the filtrate was distilled at normal pressure to remove most of the dichloromet...
Embodiment 3
[0065] Preparation of prepolymer A: four-arm polytetrahydrofuran polyol (7.5g, M n =1500g / mol), 21.40g of 3-(triethylamino)-6-caprolactone and 25mg of stannous octoate were dissolved in 25mL of N,N-dimethylformamide, and argon gas was introduced for 5 minutes to remove oxygen. The temperature of the oil bath was raised to 75°C, the reaction time was 40h, and then lowered to room temperature, settled three times with 150mL of methanol, filtered with suction, and vacuum-dried at 40°C to constant weight to obtain prepolymer A.
[0066] Preparation of the polymer shown in formula Ⅰ: 11.56g of prepolymer A, 2.02g of carboxymethoxyphosphorylcholine and 0.98g of dimethylaminopyridine were dissolved in 20mL of dichloromethane and 1.65g of N,N'-dicyclohexyl Carboimide was protected by dry argon, and the reaction was carried out under magnetic stirring at room temperature for 24 hours. Suction filtration, the filtrate was distilled at normal pressure to remove most of the dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com