Triblock polymer, drug-loaded nano-micelle, nano-drug as well as preparation method and application of nano-drug
A drug-loading nanometer and nanomedicine technology, applied in the field of nanomedicine, can solve the problem of inability to accurately deliver multi-target drugs, achieve high drug loading rate and stability, and achieve the effect of precise regulation and control of the structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of Example 1 Cyclodextrin Polypeptide Conjugate
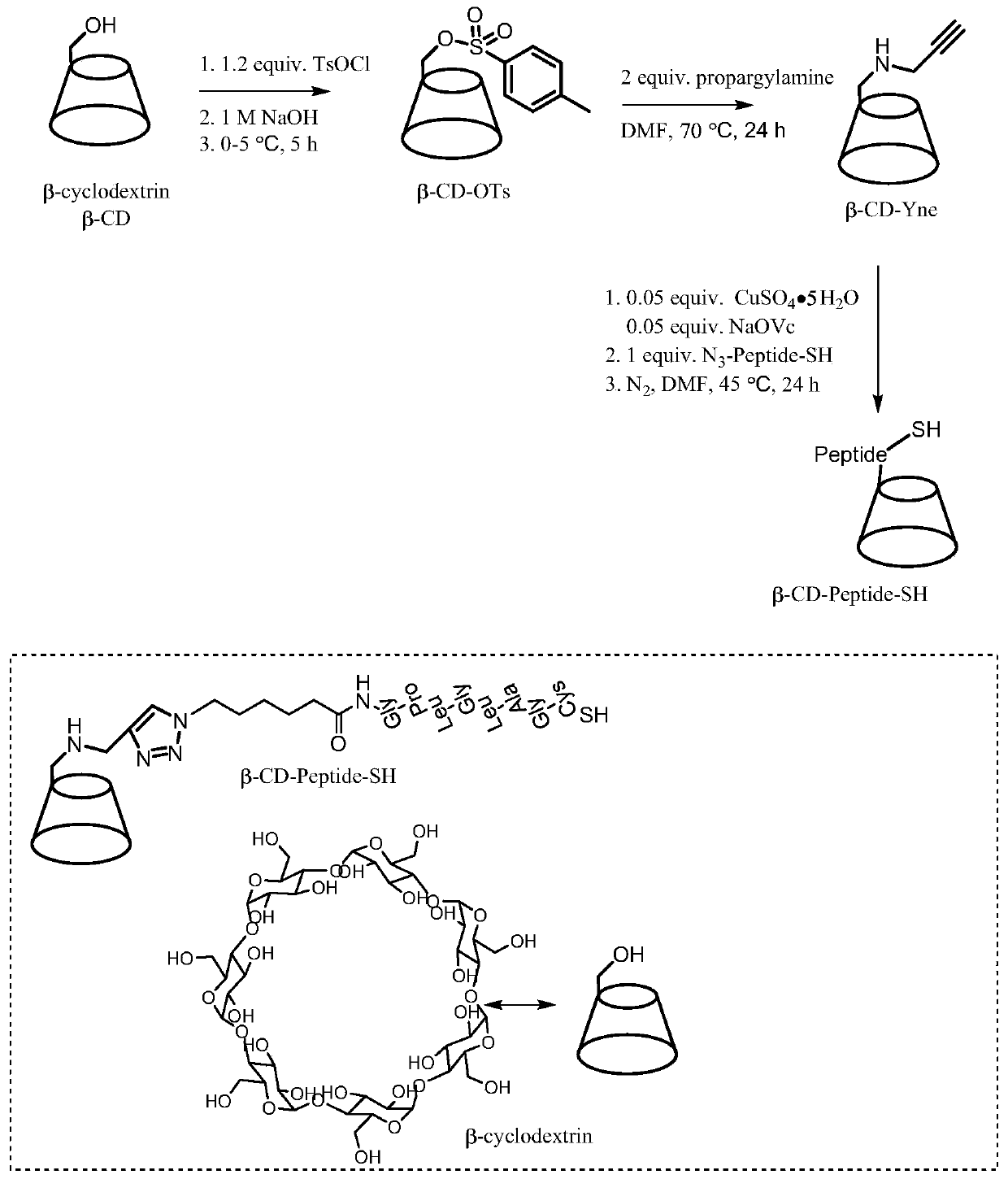
[0054] Synthesis of cyclodextrin-peptide conjugates as figure 1 As shown, it is divided into the following three parts:
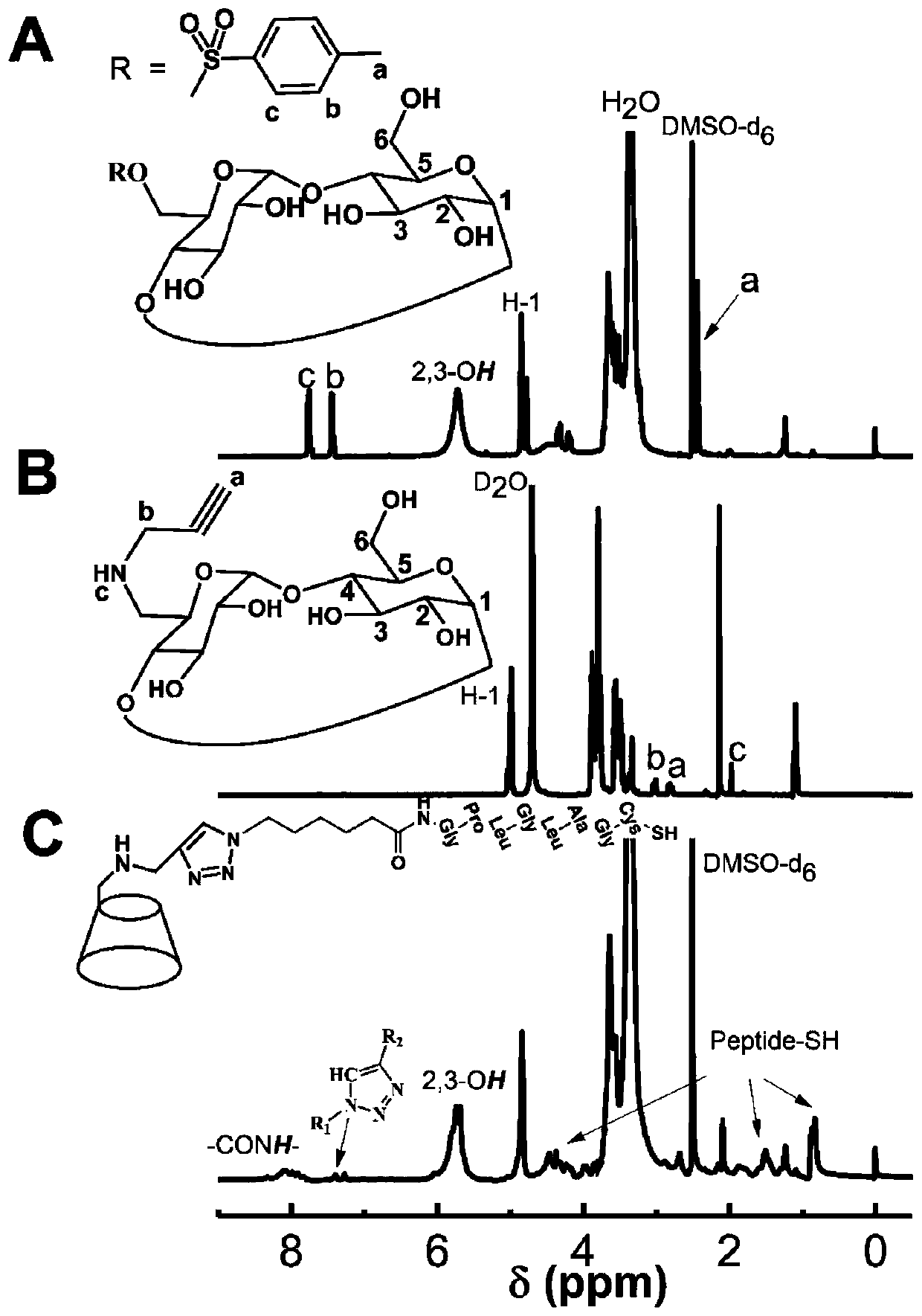
[0055] First, weigh 30g of β-cyclodextrin, dissolve it in 300mL of 1mol / L NaOH solution, add 6g of p-toluenesulfonyl chloride in an ice-water bath, and continue the magnetic stirring reaction at 0-5°C for 5h. After the reaction is complete, filter with suction , to remove solid insolubles. The filtrate was adjusted to pH=7 with 10% hydrochloric acid, and a large amount of white precipitate was produced at this time, and then it was put into a refrigerator at 4°C overnight to allow the precipitate to fully separate out. Then, the solid was obtained by suction filtration and washed three times with a small amount of cold deionized water, and the solid was recrystallized in hot water to obtain the product CD-OTs. like image 3 Shown in A, its proton nuclear magnetic resonance spectrum in de...
Embodiment 2
[0058] The synthesis of embodiment 2 related polymers
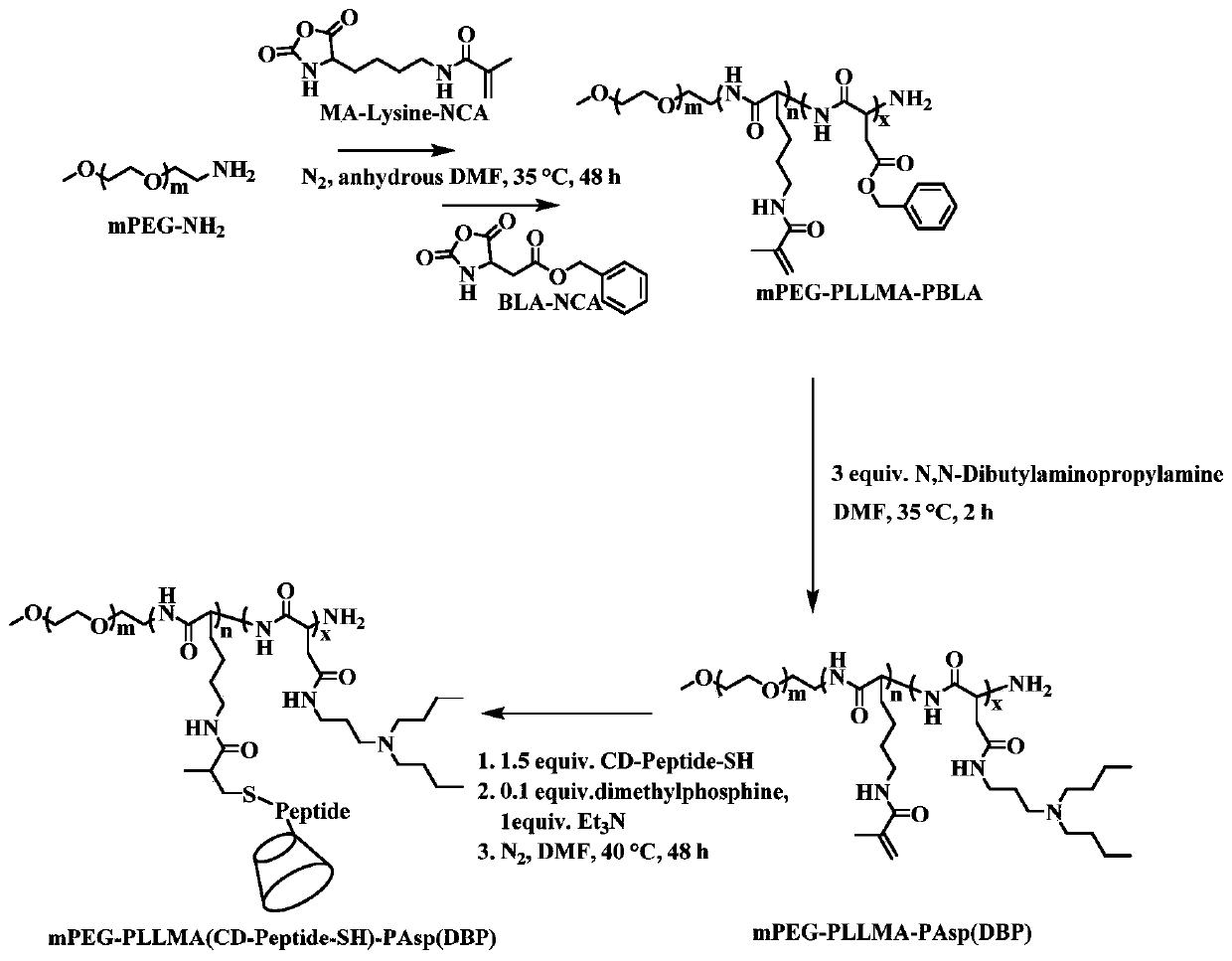
[0059] like figure 2 As shown, the synthesis of polymer (i.e. triblock polymer) is divided into the following steps:
[0060] First, 1g mPEG 5k -NH 2 (Amino conversion rate of 95%) was put into a 100 mL Schlenk tube, dried under vacuum at 70° C. for 2 h, and then cooled to room temperature. Ice water bath, filled with N 2 Under protection, add 20 mL of freshly distilled CHCl 3 , Weigh 0.5g of MA-Lysine-NCA and dissolve it in 5mL of ultra-dry DMF, and add it to the reaction bottle at one time. The reaction was sealed, and the magnetic stirring was carried out at 35° C. for 48 h (at this time, sampling was used for nuclear magnetic measurement to determine the number of PLLMA repeating units), and then in N 2 Under protection, dissolve 1g of BLA-NCA monomer in 5mL of ultra-dry DMF and add it to the above reaction flask, and add 10mL of freshly distilled CHCl 3 , sealed, and continued to react at 35°C for 48h. After...
Embodiment 3
[0066] Preparation of Example 3 Empty Carrier Nanoparticles
[0067] This example provides a method for preparing empty carrier nanoparticles (ie, drug-loaded nanomicelles responsive to the tumor microenvironment), as follows.
[0068] Dissolve a certain amount of polymer mPEG-PLLMA(CD-Peptide-SH)-PAsp(DBP) in an appropriate amount of good solvent DMF, and then add the polymer solution dropwise to a large amount of selective solvent H under stirring or ultrasonic dispersion conditions. 2 O middle. The specific operation is as follows, 20 mg of polymer is dissolved in 2 mL of DMF, 20 mL of deionized water is placed in a small beaker and placed in an ice-water bath, and the polymer solution is added dropwise to the deionized water under ultrasonic vibration. After all the addition was completed, the micellar solution was put into a dialysis bag and dialyzed with deionized water for 2 days to remove DMF. After the organic solvent is removed by dialysis, it is concentrated by ul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com