CoMn-P-NCNT multifunctional catalyst as well as a preparation method and application thereof
A comn-p-ncnt, multifunctional technology, applied in the field of CoMn-P-NCNT multifunctional catalyst and its preparation, can solve the problems of poor activity, limited to powder materials, incomplete functions, etc. Catalytic performance, low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
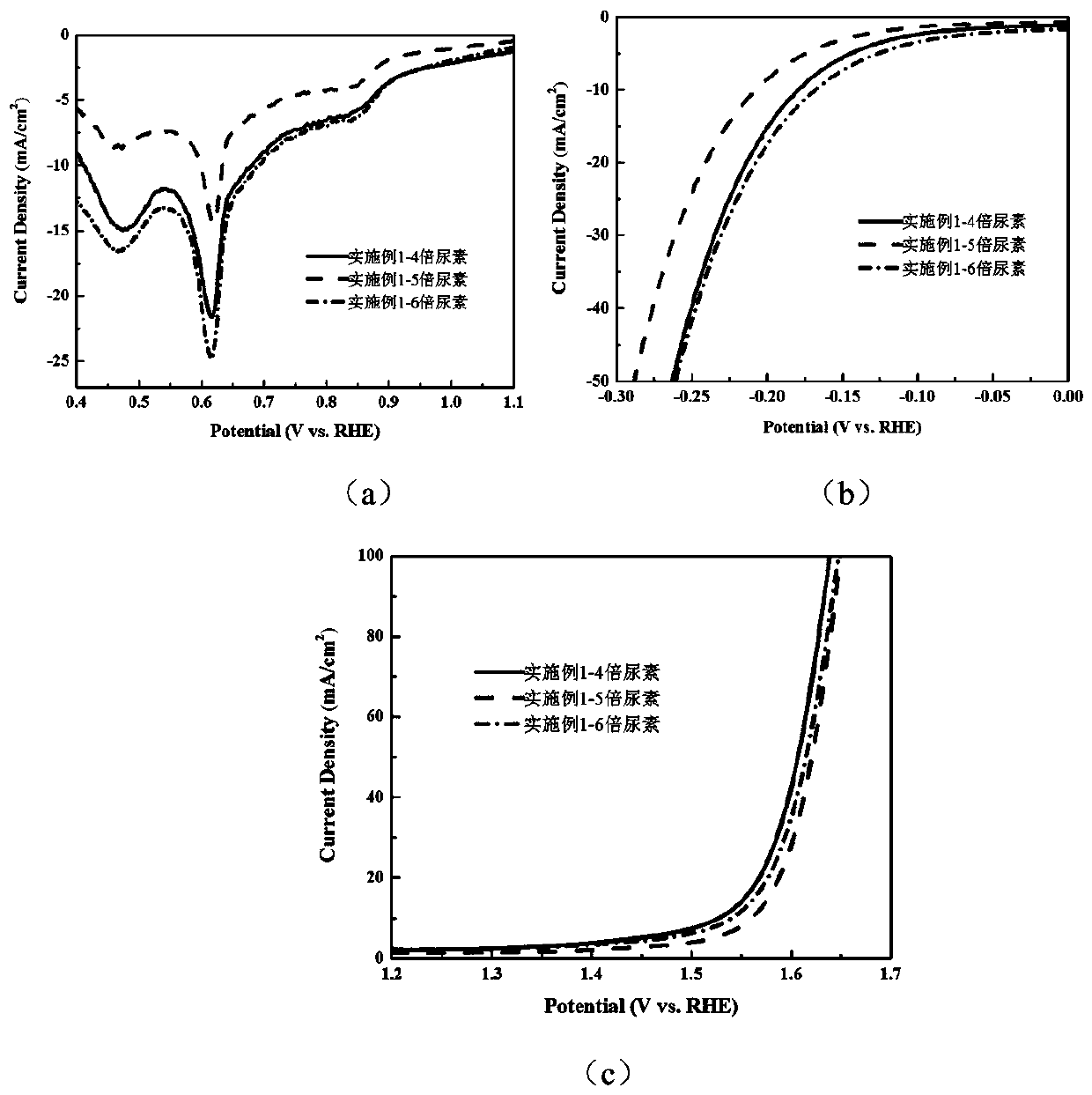
[0042] Example 1 CoMn-NCNT multifunctional catalysts with different urea contents were prepared by the following method.
[0043] Preparation of precursors: cobalt chloride 1.167mmol, manganese chloride 2.333mmol, ammonium fluoride 7mmol, and the amount of urea were 14mmol (the molar ratio of urea to metal salt was 4), 17.5mmol (the molar ratio of urea to metal salt The ratio is 5), 21mmol (the molar ratio of urea to metal salt is 6), and the concentration of dopamine hydrochloride is 20mmol / L. Dissolve the above substances in a 100mL beaker, add 70mL deionized water, and stir well. Put the carbon paper into the Teflon liner and pour the solution prepared above. After hydrothermal reaction at 100°C for 10 hours, it was washed and dried to obtain a precursor.
[0044] Then, the above-prepared precursor and 14 g of urea were annealed at a heating rate of 5° C. / min to 700° C. for 2 hours to obtain the CoMn-NCNT multifunctional catalyst.
[0045] figure 1 The middle is the effe...
Embodiment 2
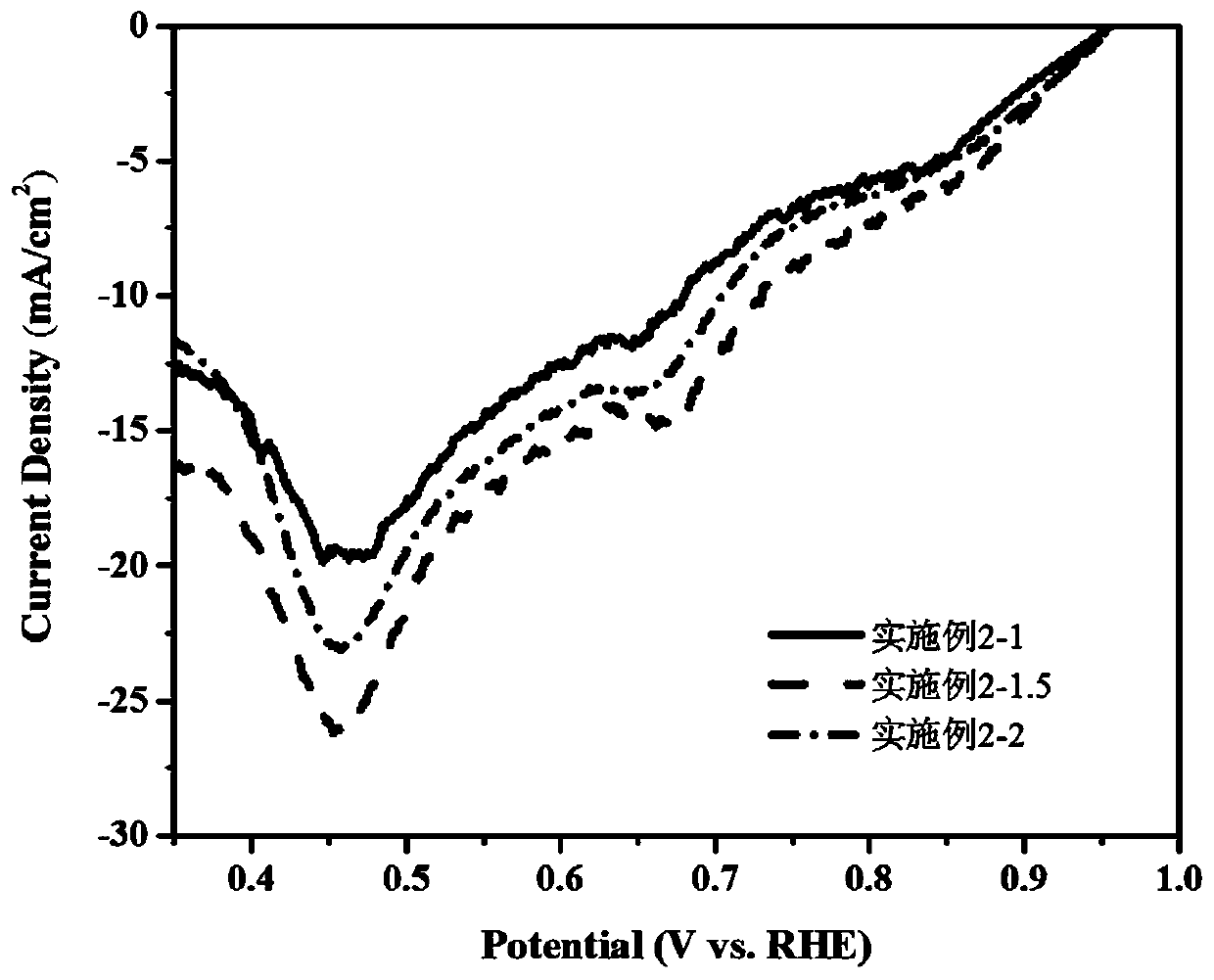
[0047] Example 2 CoMn-P-NCNT multifunctional catalysts with different sodium hypophosphite contents were prepared by the following method.
[0048] Preparation of precursor: cobalt chloride 1.167mmol, manganese chloride 2.333mmol, ammonium fluoride 7mmol, the amount of urea is 21mmol, the concentration of dopamine hydrochloride in the mixed system is 20mmol / L, add sodium hypophosphite 3.5mmol ( Sodium hypophosphite is 1 times the amount of metal salt substance), 5.25mmol (sodium hypophosphite is 1.5 times the amount of metal salt substance), 7mmol (sodium hypophosphite is 2 times the amount of metal salt substance, and the metal salt is The amount of the total substance of cobalt chloride and manganese chloride), the above substances were dissolved in a 100mL beaker, 70mL deionized water was added, and stirred evenly. Put the carbon paper into the Teflon liner and pour the solution prepared above. Hydrothermal reaction was carried out at 100°C for 10 hours. Wash, tumble dry....
Embodiment 3
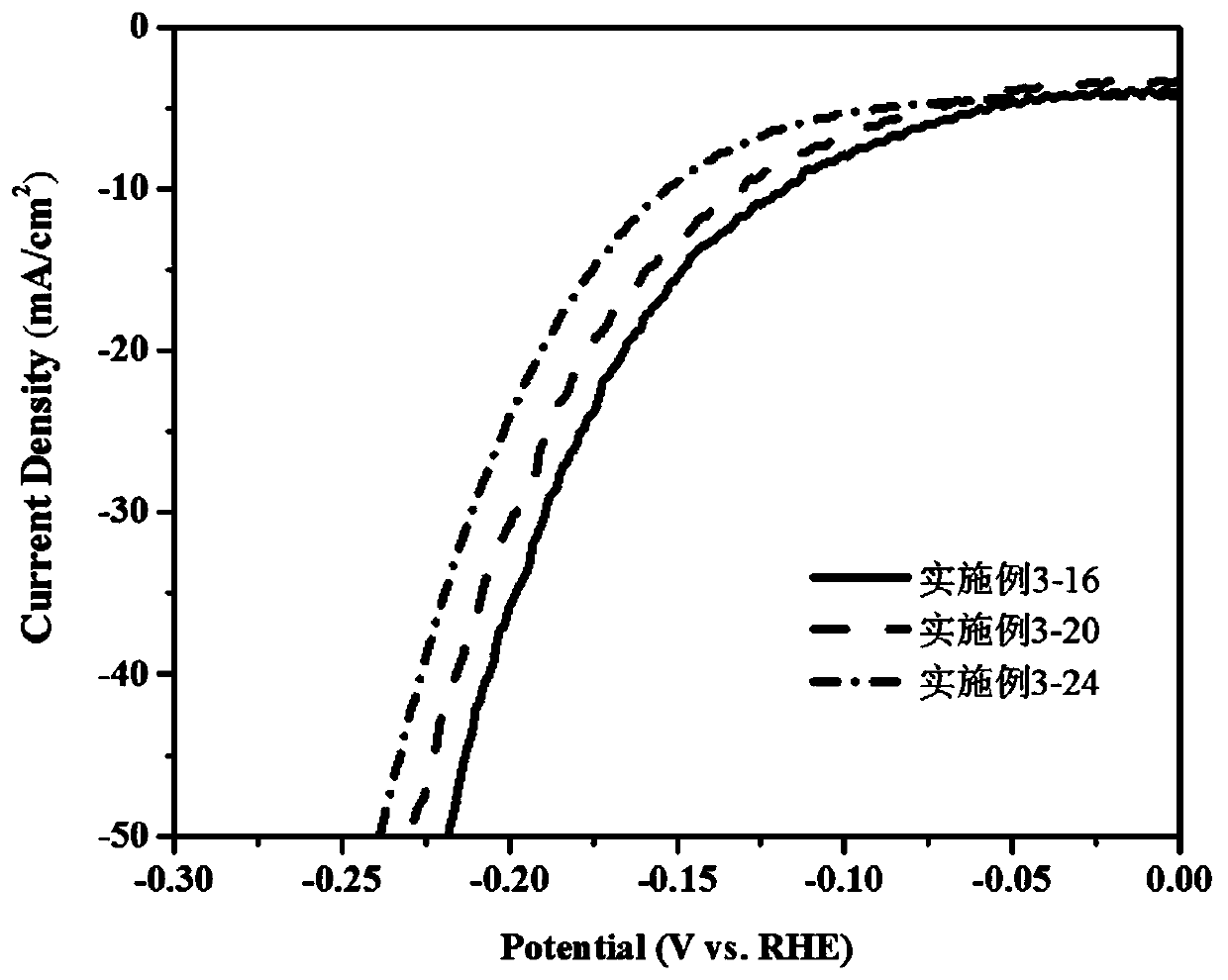
[0051] Example 3 CoMn-P-NCNT multifunctional catalysts with different contents of dopamine hydrochloride were prepared by the following method.
[0052] Preparation of precursors: cobalt chloride 1.167mmol, manganese chloride 2.333mmol, ammonium fluoride 7mmol, the amount of urea is 21mmol, the concentration of dopamine hydrochloride in the mixed system is respectively (16mmol / L, 20mmol / L, 24mmol / L), the amount of phytic acid substance is 5.25mmol, dissolve the above substance in a 100mL beaker, add 70mL deionized water, and stir evenly. Put the carbon paper into the Teflon liner and pour the solution prepared above. Hydrothermal reaction was carried out at 100°C for 10 hours. Wash, tumble dry.
[0053] Then, the above-prepared precursor and 14 g of urea were annealed at a heating rate of 5° C. / min to 700° C. for 2 hours to obtain the CoMn-P-NCNT multifunctional catalyst.
[0054] image 3 The in-situ HER polarization curve figure of the CoMn-P-NCNT multifunctional cataly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Limiting current density | aaaaa | aaaaa |
| Limiting current density | aaaaa | aaaaa |
| Limiting current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com