Continuous reaction device and method for preparing aldehyde through hydroformylation reaction
A reaction device and chemical reaction technology, applied in the field of continuous reaction devices, can solve the problems of catalyst poisoning and deactivation, large energy consumption, and high energy consumption of the overall process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
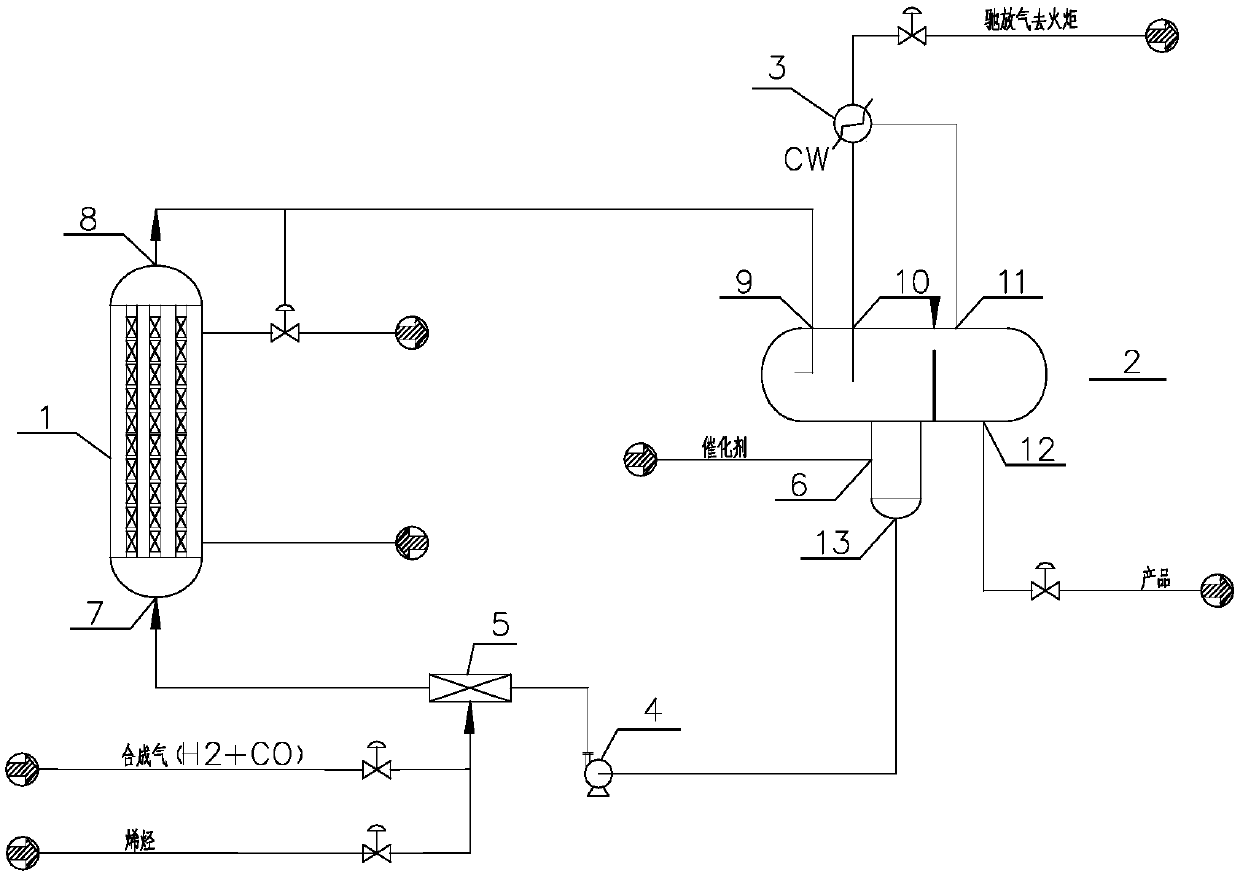
[0122] use figure 1 As shown in the process flow, the process conditions are as follows:
[0123] The catalyst aqueous solution adopts the proportioning composition of Example 1 in the published patent number CN101462932A.
[0124] Reaction temperature: 80°C, reaction pressure is 2.5MPa (A);
[0125] Reactor inlet conditions:
[0126] Feed flow of catalyst aqueous solution: 10m 3 / Hour;
[0127] Ethylene feed flow: 25Nm 3 / Hour;
[0128] CO+H 2 Feed flow: 50Nm 3 / Hour;
[0129] CO:H 2 = 1:1 (molar ratio);
[0130] The discharge results at the discharge port 3:
[0131] Ethylene conversion rate: 98%;
[0132] Propionaldehyde yield: 98%.
[0133] In this example, the production process of preparing propionaldehyde by hydroformylation of ethylene olefin is realized with a high ethylene conversion rate of 98% and a high propionaldehyde selectivity of 98%.
Embodiment 2
[0135] use figure 1 As shown in the process flow, the process conditions are as follows:
[0136] The catalyst aqueous solution adopts the proportioning composition of Example 5 in the published patent number CN101462932A.
[0137] Reaction temperature: 110°C, reaction pressure 2.5MPa (A);
[0138] Reactor inlet conditions:
[0139] Feed flow of catalyst aqueous solution: 10m 3 / Hour;
[0140] Propylene feed flow: 50kg / hour;
[0141] CO+H 2 Feed flow: 50Nm 3 / Hour;
[0142] CO:H 2 = 1:1 (molar ratio);
[0143] The discharge results at the discharge port 3:
[0144] Propylene conversion rate: 98%;
[0145] n-Butyraldehyde yield: 97%;
[0146] n-Butyraldehyde:Isobutyraldehyde=40:1 (molar ratio).
[0147] In this example, the production process of preparing n-butyraldehyde by hydroformylation of propene olefin is realized with a high propylene conversion rate of 98% and a high n-butyraldehyde selectivity of 97%.
Embodiment 3
[0149] use figure 1 As shown in the process flow, the process conditions are as follows:
[0150] The catalyst aqueous solution adopts the proportioning composition of Example 10 in the published patent number CN101462932A.
[0151] Reaction temperature: 120°C, reaction pressure is 3.0MPa (A);
[0152] Reactor inlet conditions:
[0153] Feed flow of catalyst aqueous solution: 10m 3 / Hour;
[0154] 1-Butene feed flow: 60kg / hour;
[0155] CO+H 2 Feed flow: 50Nm 3 / Hour;
[0156] CO:H 2 = 1:1 (molar ratio);
[0157] The discharge results at the discharge port 3:
[0158] 1-Butene conversion rate: 97%;
[0159] n-valeraldehyde yield: 97%;
[0160] n-valeraldehyde:isovaleraldehyde = 60:1 (molar ratio).
[0161] In this example, the production process of preparing n-valeraldehyde by hydroformylation of 1-butene is realized with a high 1-butene conversion rate of 97% and a high n-valeraldehyde selectivity of 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com