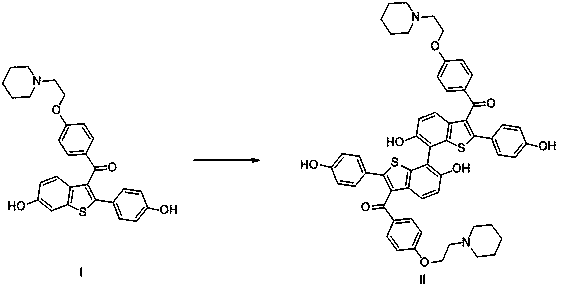
Preparation and separation method of raloxifene dimer
A dimer, liquid phase preparation technology, applied in the field of medicine, can solve the problems of high price of SIN-1, limited production, difficult to separate and obtain pure raloxifene dimer, etc., and achieves low cost and environmental friendliness. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of crude product of raloxifene dimer
[0030] Synthetic route such as figure 1 shown.
[0031] Step 1. Add 5g of raloxifene, 150mL of methanol, 5mL of ethylenediamine, and 15mL of 30% hydrogen peroxide into the reaction bottle, stir in an ice bath, and cool to 0~5°C;
[0032] Step 2, add a total of 3.25g of ferric chloride, and control the temperature not to exceed 50°C;
[0033] Step 3, after adding ferric chloride, reflux and stir for 1 hour;
[0034] Step 4. Concentrate the reaction solution under reduced pressure at 20~25°C, add 25mL of methanol and 25mL of acetone, stir well and then filter with suction;
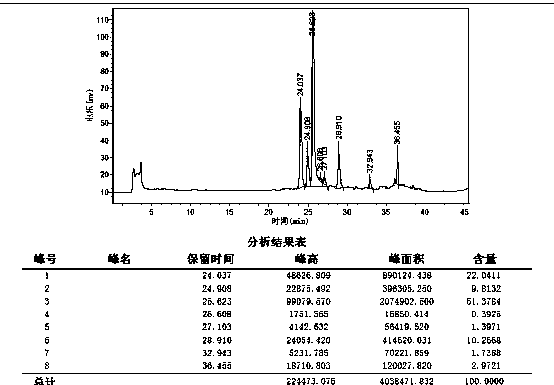
[0035] Step 5. Add the filtered solid to 6M hydrochloric acid, stir evenly, filter with suction, wash the filter cake once with 25 mL of water, and dry at 50°C under normal pressure to obtain 4.8 g of crude raloxifene dimer, as figure 2 As shown, the HPLC purity of raloxifene dimer in the crude product was 51.3% (25.6min).
[0036] The...
Embodiment 2
[0044] Example 2 Preparation of refined product of raloxifene dimer
[0045] Step 6. Purifying the crude raloxifene dimer above by preparative liquid chromatography, and collecting the preparation solution containing raloxifene dimer;
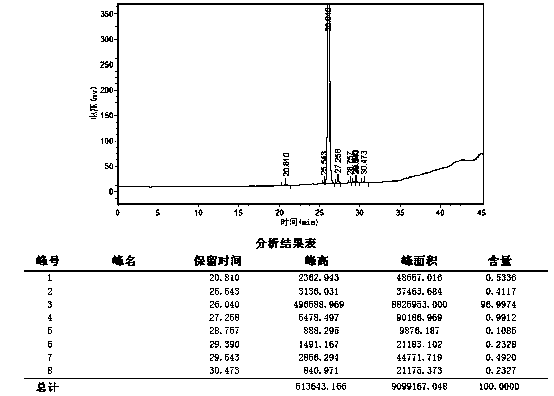
[0046] Step 7. Concentrate the collected preparation solution to dryness under reduced pressure at 20°C, add water, stir evenly, filter with suction, wash the filter cake once with water, and dry 1.9g of refined raloxifene dimer at 50°C under normal pressure ,Such as image 3 As shown, the HPLC purity of the refined product is 96.9%.
[0047] Preparative chromatographic purification conditions are:
[0048] Chromatographic column: C18 50×500mm 10µm;
[0049] Flow rate: 35mL / min;
[0050] Column temperature: 25°C;
[0051] Wavelength: 280nm;
[0052] Gradient elution conditions are shown in the table below:
[0053]
[0054] Wherein A is acetonitrile, and the mobile phase B is 0.02M potassium dihydrogen phosphate, and the pH is adjuste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com