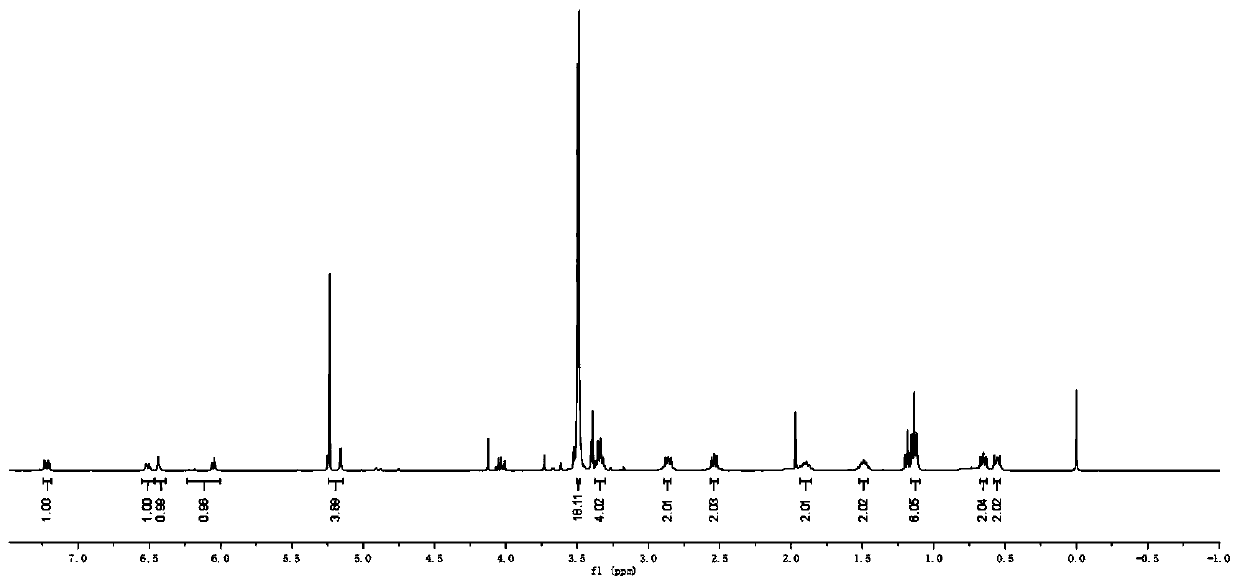
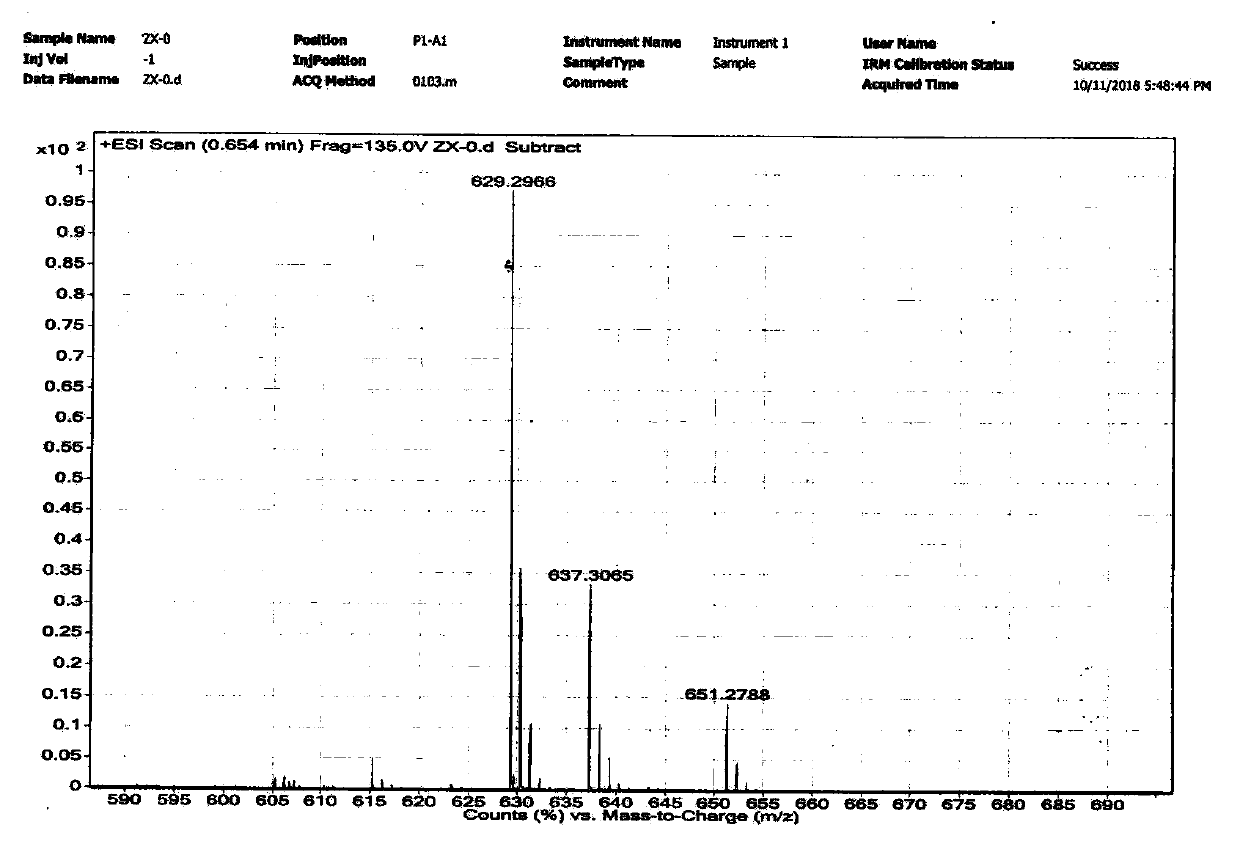
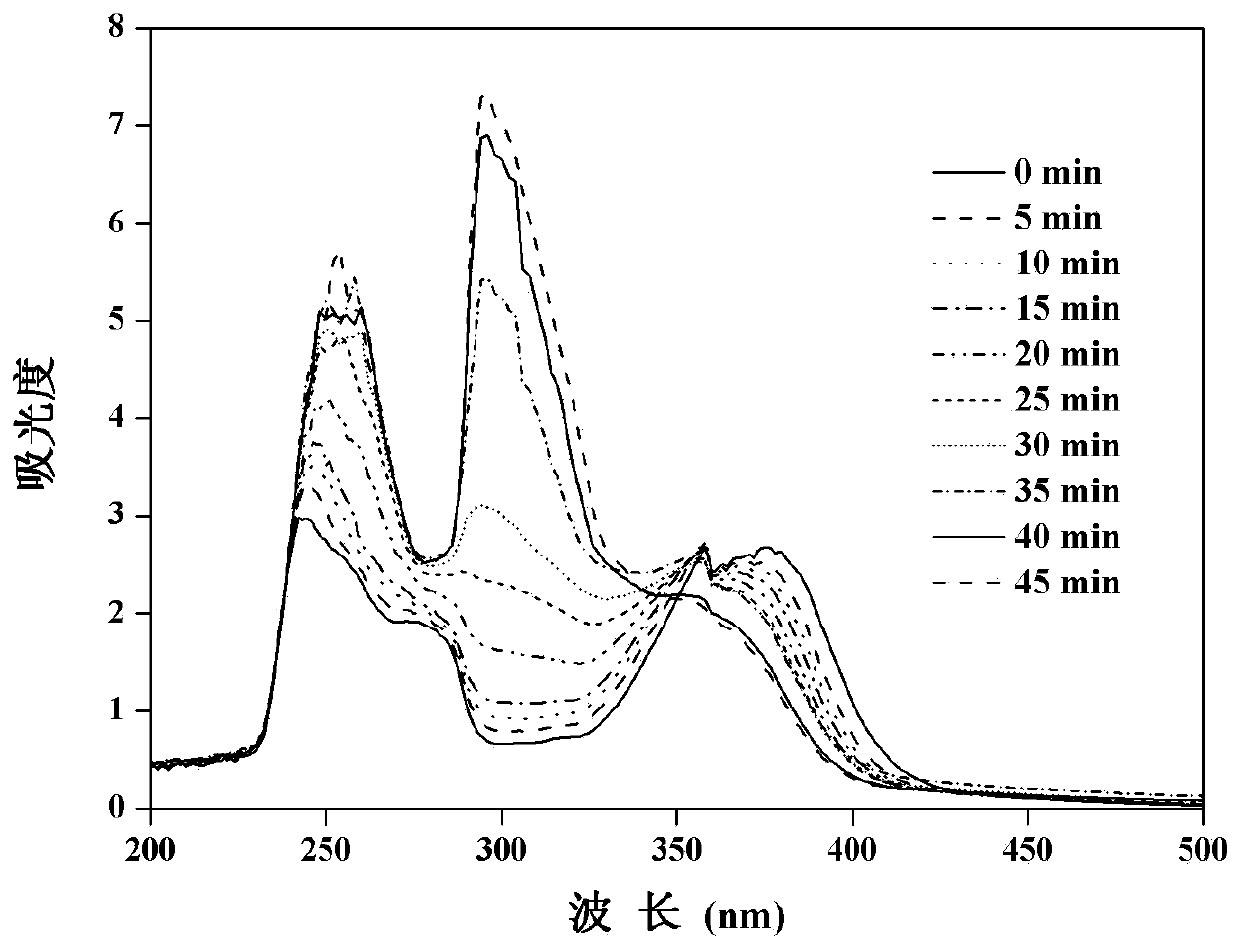
Coumarin-based bridged silane and preparation method thereof
A coumarin-based, bisilane technology is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc. The effect of mild, good light response properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A preparation method of coumarin-based bridged silane, comprising steps as follows:
[0062] (1) Dissolve 7-N,N-diethylamino-4-methylcoumarin (4.63g, 20mmol) in 120mL 1,4-dioxane, add selenium dioxide ( 3.33 g, 30 mmol). The reaction mixture was heated to 90°C under vigorous stirring and reacted for 48h. After the reaction, the reaction system was filtered and rotary evaporated to obtain the intermediate product 1, which was dissolved in 130mL of methanol, and sodium borohydride (1.52g, 40mmol) was added in five batches under the condition of 0-4°C ice-water bath . Then the reaction system was raised to room temperature and stirred at room temperature for 8 h. Carefully add 40mL of 1mol / L HCl solution to the reaction system for hydrolysis, and extract the obtained red solution three times with 20mL of anhydrous dichloromethane, combine the organic phases, and use 30mL deionized water and 30mL saturated sodium bicarbonate solution for the organic phases in sequence a...
Embodiment 2
[0070] A preparation method of coumarin-based bridged silane, comprising steps as follows:
[0071] (1) Dissolve 7-N,N-diethylamino-4-methylcoumarin (4.63g, 20mmol) in 120mL 1,4-dioxane, add selenium dioxide ( 3.33 g, 30 mmol). The reaction mixture was heated to 85°C under vigorous stirring and reacted for 36h. After the reaction, the reaction system was filtered and rotary evaporated to obtain the intermediate product 1, which was dissolved in 130mL of methanol, and sodium borohydride (1.90g, 50mmol) was added in five batches under the condition of 0-4°C ice-water bath . Afterwards, the reaction system was raised to room temperature, and the reaction was stirred at room temperature for 4 h. Carefully add 30mL of 1mol / L HCl solution to the reaction system for hydrolysis, and extract the obtained red solution three times with 20mL of anhydrous dichloromethane, combine the organic phases, and use 30mL of deionized water and 30mL of saturated sodium bicarbonate solution in tur...
Embodiment 3
[0077] A preparation method of coumarin-based bridged silane, comprising steps as follows:
[0078] (1) Dissolve 7-N,N-diethylamino-4-methylcoumarin (4.63g, 20mmol) in 120mL 1,4-dioxane, add selenium dioxide ( 2.22g, 20mmol). The reaction mixture was heated to 80°C under vigorous stirring and reacted for 24h. After the reaction was completed, the reaction system was filtered and rotary evaporated to obtain an intermediate product 1, which was dissolved in 130 mL of methanol, and sodium borohydride (1.14 g, 30 mmol) was added in three batches under the condition of an ice-water bath at 0-4°C . Afterwards, the reaction system was raised to room temperature, and the reaction was stirred at room temperature for 4 h. Carefully add 40mL of 1mol / L HCl solution to the reaction system for hydrolysis, and extract the obtained red solution three times with 20mL of anhydrous dichloromethane, combine the organic phases, and use 30mL deionized water and 30mL saturated sodium bicarbonate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com