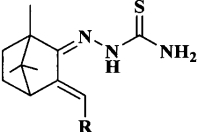
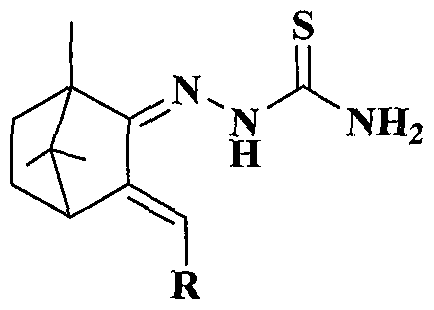
Preparation and antitumor activity of camphor-based thiosemicarbazone compounds
A technology of thiosemicarbazones and compounds, which is applied in antineoplastic drugs, organic chemistry, drug combinations, etc., can solve the problems of high toxicity in clinical application and chemotherapy side effects, and achieve the effect of abundant sources and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
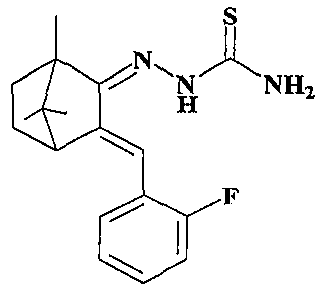
[0028] Preparation of 2-(3-(2′-fluorobenzylidene)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene)hydrazinethiocarboxamide (Compound 1):
[0029]
[0030] In a 50ml three-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, add thiosemicarbazide (2mmol) and ethanol (35mL) in sequence, start stirring to dissolve the thiosemicarbazide, add 3-(2'-fluoro After benzylidene)camphor (2mmol), the temperature was raised to 90°C, 3 drops of concentrated hydrochloric acid were added dropwise, and the reflux reaction was continued for 48 hours (TLC tracking detection). After the reaction, the reaction solution was concentrated to remove ethanol to obtain a yellow crude product. The crude product was purified by column chromatography (ethyl acetate: petroleum ether = 1:6, 1% TEA) to obtain a light yellow solid powder. Yield 78.9%; m.p.156.1~156.5℃; 1 H NMR (400MHz, CDCl 3 ): δ9.22(s, 1H), 7.36(s, 1H), 7.25-7.32(m, 2H), 7.23(s, 1H), 7.07-7.18(m, 2H), 6.40(s, 1...
Embodiment 2
[0032] Preparation of 2-(3-(4′-fluorobenzylidene)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene)hydrazinethiocarboxamide (Compound 2):
[0033]
[0034] The preparation method is the same as in Example 1. Substitute 2-fluorobenzaldehyde with 4-fluorobenzaldehyde to obtain light yellow solid powder. Yield 79.4%; m.p.192.5~192.9℃; 1 H NMR (400MHz, CDCl 3 ): δ9.18(s, 1H), 7.30(t, J=4Hz, 2H), 7.27(s, 1H), 7.21(s, 1H), 7.07(t, J=8Hz, 2H), 6.40(s , 1H), 2.86(d, J=4Hz, 1H), 2.05-2.10(m, 1H), 1.77-1.87(m, 1H), 1.53(d, J=12Hz, 1H), 1.04(s, 3H) , 0.91(s, 3H), 0.75(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ179.92, 161.20, 158.90, 141.22, 132.18, 130.86, 128.72, 115.72, 54.17, 51.71, 47.97, 33.42, 25.77, 20.01, 18.19, 11.28; HR-MS (ESI): m / z calculated for C 18 h 22 FN 3 S, [M+H] + 332.1597, found 332.1591.
Embodiment 3
[0036] Preparation of 2-(3-(2′-chlorobenzylidene)-1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene)hydrazinethiocarboxamide (compound 3):
[0037]
[0038] The preparation method is the same as in Example 1. Substitute 2-chlorobenzaldehyde for 2-fluorobenzaldehyde to obtain light yellow solid powder. Yield 82.6%; m.p.199.7~200.5℃; 1 H NMR (400MHz, CDCl 3 ): δ9.24(s, 1H), 7.41-7.43(m, 1H), 7.39(s, 1H), 7.32(s, 1H), 7.26(t, J=4Hz, 2H), 7.21-7.24(m , 1H), 6.46(s, 1H), 2.66(d, J=4Hz, 1H), 1.97-2.03(m, 1H), 1.81-1.86(m, 1H), 1.51(d, J=8Hz, 2H) , 1.05(s, 1H), 0.89(s, 1H), 0.80(s, 1H);13 C NMR (100MHz, CDCl 3 ): δ 179.96, 158.11, 142.78, 134.71, 133.85, 130.24, 129.62, 129.33, 126.77, 117.60, 54.37, 51.67, 47.75, 33.36, 25.90, 20.07, 18.12, 11.26 for C 18 h 22 ClN 3 S; [M+H] + 348.1301, found 348.1297.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com