Bismuth-doped solid oxide battery fuel electrode material and preparation method and application thereof
A solid oxide, battery fuel technology, applied in solid electrolyte fuel cells, battery electrodes, chemical instruments and methods, etc., can solve the problems of SOC industrialization requirements are still far away, low catalytic performance, etc., and achieve good chemical and structural stability. , The effect of enhanced catalytic activity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Take the citrate combustion method as an example below to introduce the synthetic method of the material of the present invention, and the concrete implementation steps are:
[0048] a, with La 2 o 3 , Sr(NO 3 ) 2 , Cr(NO 3 ) 3 9H 2 O, Bi(NO 3 ) 3 ·5H 2 O, Fe(NO 3 ) 3 9H 2 O is raw material, according to La 1-x-z Sr x Bi z Cr 1-y Fe y o 3-δ , wherein 0<x<1.0, 0<y<1.0, 0<z≤0.5 stoichiometric ratio is dissolved in dilute nitric acid to obtain a raw material solution;
[0049] b. Using citric acid and ethylenediaminetetraacetic acid as a complexing agent, mixing with the above-mentioned raw material solution to obtain a mixed solution; the molar ratio of the citric acid, ethylenediaminetetraacetic acid and the total amount of metal ions is preferably 1:1.5: 1;
[0050] c. Aqueous ammonia can be added dropwise to the above mixed solution, the pH of the mixed solution is adjusted to 5-6, and then preferably stirred for 1 hour-4 hours to obtain a uniform so...
Embodiment 1
[0071] Embodiment 1 adopts citrate combustion method to prepare La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ Powder
[0072] Press La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ The stoichiometric ratio of the raw material La 2 o 3 , Sr(NO 3 ) 2 , Cr(NO 3 ) 3 9H 2 O, Bi(NO 3 ) 3 ·5H 2 O, Fe(NO 3 ) 3 9H 2 O is dissolved in dilute nitric acid, and citric acid and ethylenediaminetetraacetic acid are added as complexing agents, and then the pH of the solution is adjusted to about 6 with ammonia water, and the molar ratio of citric acid, ethylenediaminetetraacetic acid and the total amount of metal ions is 1: 1.5:1; put the above uniformly stirred solution on a heating platform and heat it until spontaneous combustion occurs, collect the powder obtained after combustion, grind it, and then calcinate it in a muffle furnace at 1000°C for 3 hours to obtain La 0.65 Sr0 .25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ Powder.
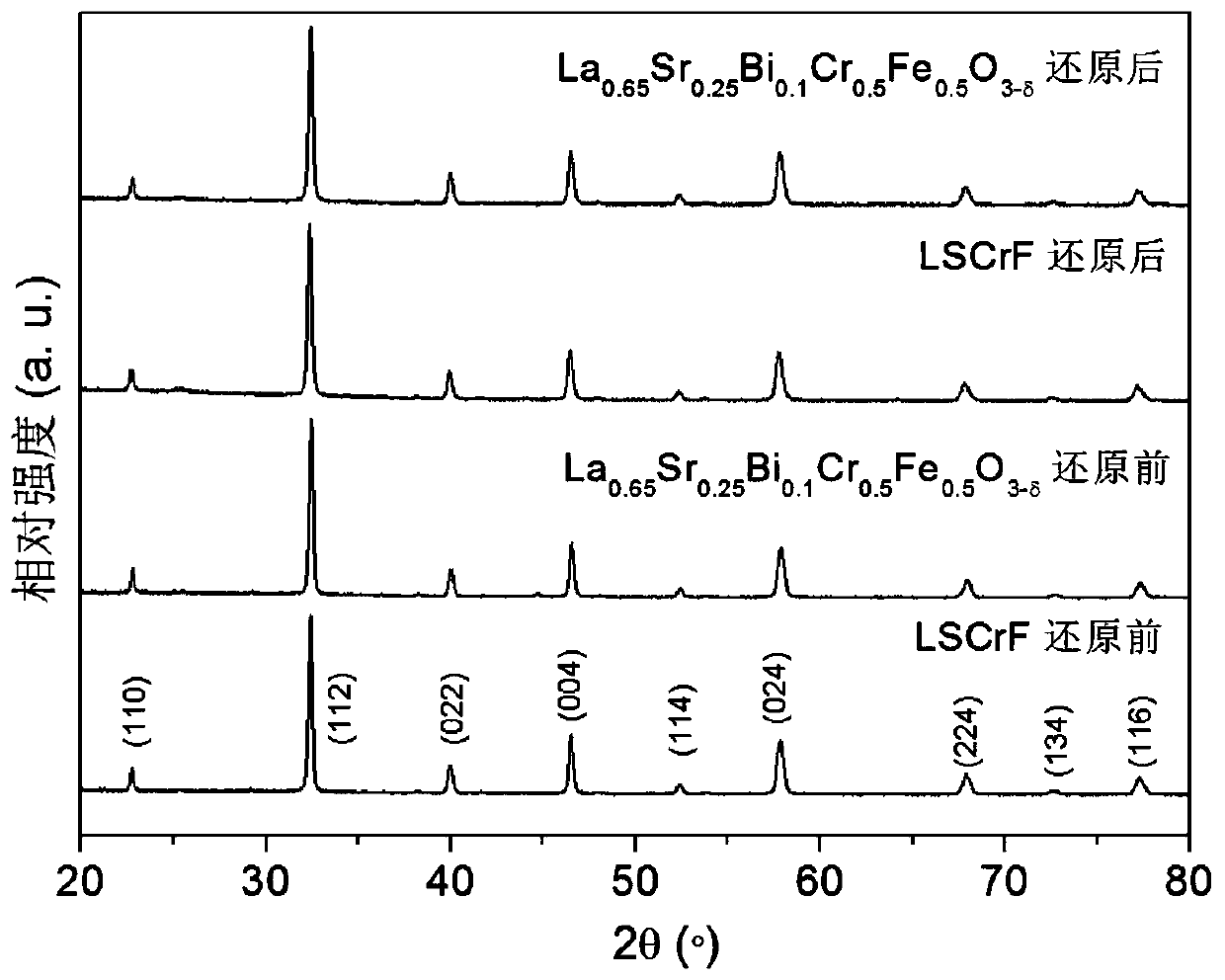
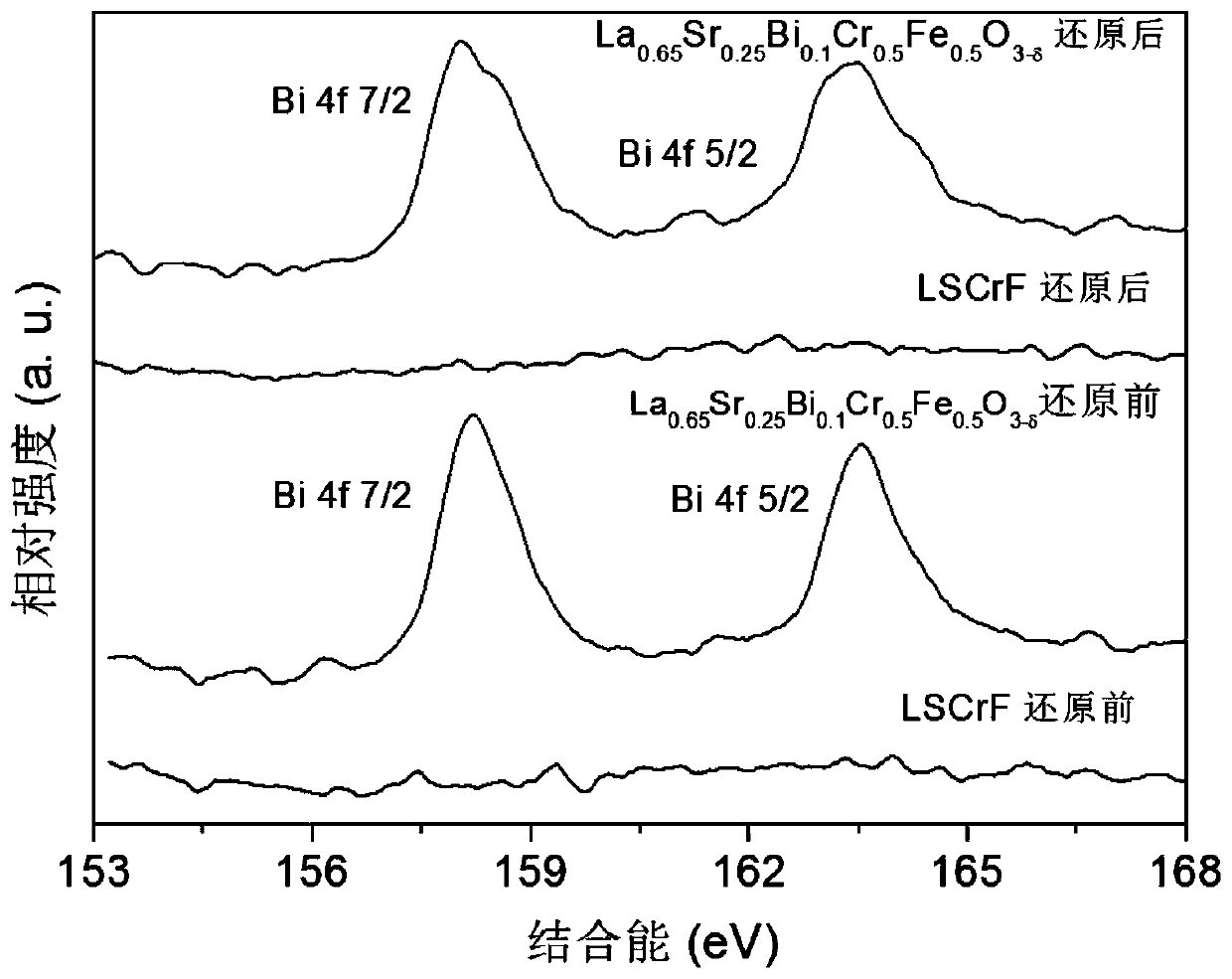
[0073] La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3...
Embodiment 2
[0076] Example 2La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ Conductivity Measurement of Perovskite Oxide Fuel Electrode Materials
[0077] La in embodiment 1 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ The powder and 5wt.% polyvinyl alcohol binder are added into the powder 4-5 drops per gram of powder to mix and grind evenly, then dry pressed into strip samples, and sintered in a high-temperature furnace at 1400 ° C for 10 hours to obtain Dense samples were then tested for electrical conductivity using the four-probe method.
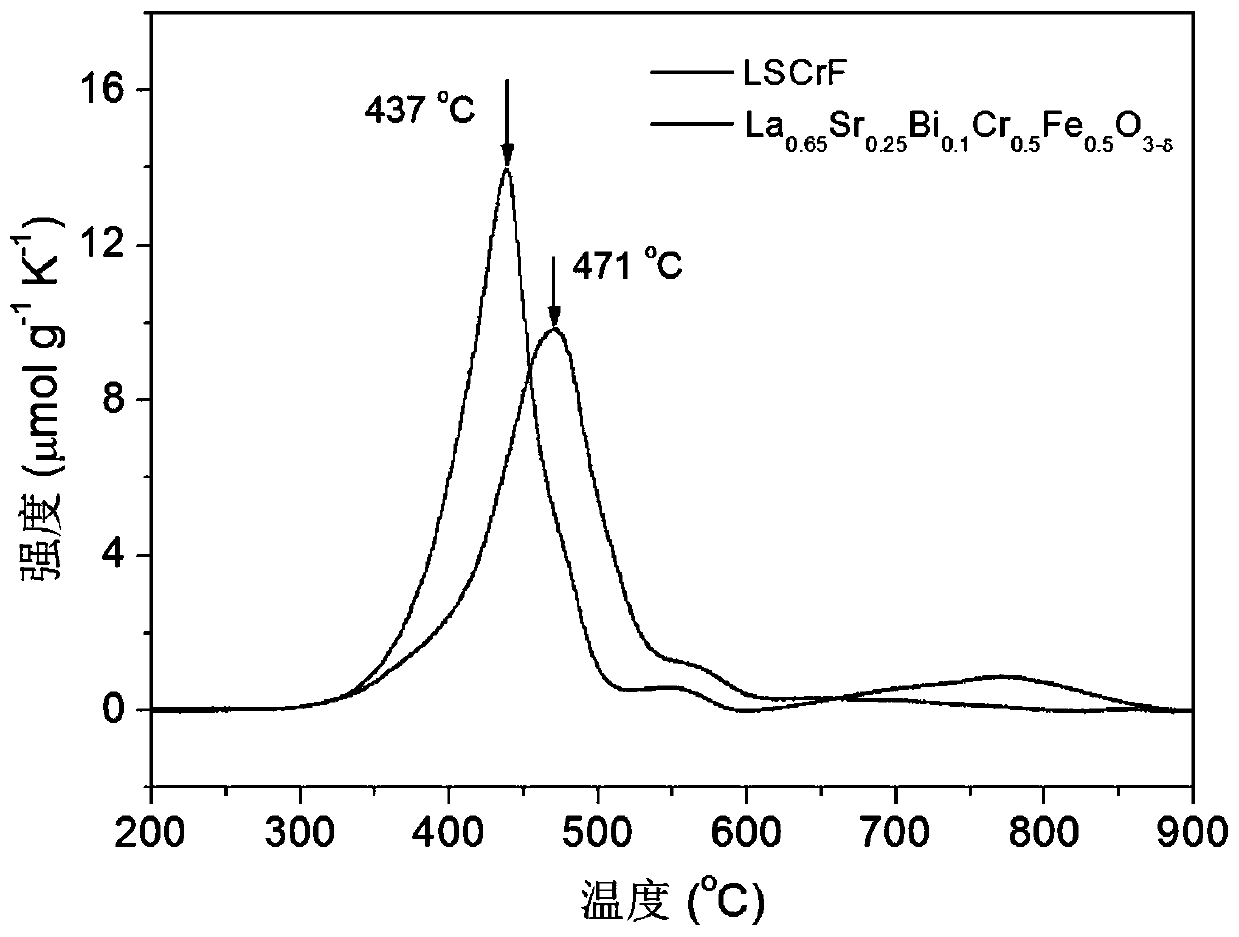
[0078] La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ The conductivity of the sample under air and hydrogen is as Figure 4 shown; under humid hydrogen conditions at 600–850°C, La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ The electronic conductivity of 0.028–0.21S cm -1 , which is 2–3 times higher than that of LSCrF under the same conditions; in an air atmosphere of 600–850°C, La 0.65 Sr 0.25 Bi 0.1 Cr 0.5 Fe 0.5 o 3-δ The electronic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com