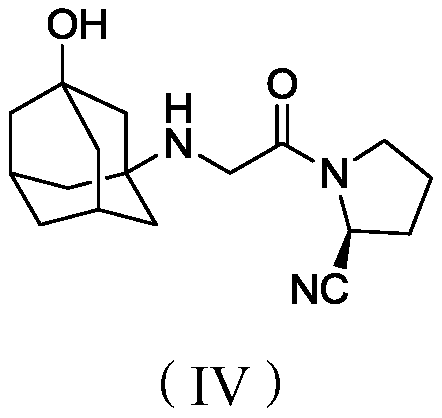
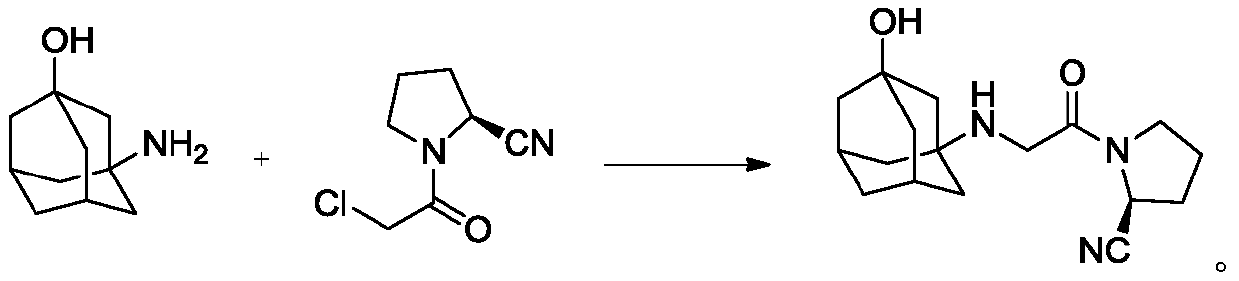
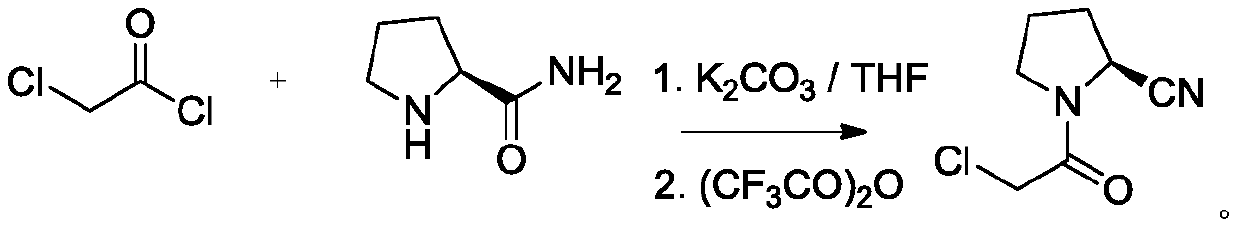
Preparation method of vildagliptin intermediate
An intermediate, proline amide technology, applied in the field of preparation of vildagliptin intermediate -1-pyrrolidine-2-amide, can solve the problems of high solubility, low solubility, large loss, etc., and achieve excellent product quality and easy operation Simple, Gentle Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 20.0g of L-prolineamide in a 250mL four-neck flask, add 200mLTHF, heat up to 40°C under stirring to dissolve L-prolineamide, cool down to 0°C after dissolving, add 20.2g of propylene oxide (2.00eq ), dropwise added 35.4g of chloroacetyl chloride (1.79eq), and gradually produced a white suspension. After 3 hours of dropwise addition, the heat preservation reaction was carried out for 2 hours, and suction filtration gave a white solid, washed with a small amount of THF, and dried in vacuo at 40°C to obtain 24.8 grams of the product. The yield was 74.4%, and the content detected by HPLC was 99.52%.
Embodiment 2
[0028] Weigh 20.0g of L-prolineamide into a 250mL four-neck flask, add 150mL of dichloromethane, heat up to 40°C under stirring to dissolve L-prolineamide, cool down to 0°C after dissolving, add 20.2g of propylene oxide (2.00eq), 35.4g chloroacetyl chloride (1.79eq) was added dropwise, and the solid produced during the dropwise addition formed a spherical shape. After the dropwise addition was completed, the heat preservation reaction was carried out for 2 hours, and the viscous solid was filtered by HPLC detection and contained a large amount of unreacted prophylamine Aminoamide, experiment failed.
Embodiment 3
[0030] Weigh 20.0g of L-prolinamide in a 250mL four-neck flask, add 150mL of acetonitrile, heat up to 40°C under stirring to dissolve L-prolineamide, cool down to 0°C after dissolving, add 20.2g of propylene oxide (2.00 eq), 35.4g chloroacetyl chloride (1.79eq) was added dropwise, and the solid produced during the dropwise addition formed a spherical shape. After the dropwise addition, the insulation reaction was carried out for 2h, and the viscous solid HPLC detected by filtration contained a large amount of unreacted prolineamide , the experiment failed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com