Photochromic coating based on organic-inorganic hybridization and preparation method of photochromic coating
A photochromic and hybrid technology, used in organic chemistry, multi-color effect coatings, polyurea/polyurethane coatings, etc., can solve the problems of low discoloration efficiency, loss of discoloration performance of coatings, unstable chemical properties of organic compounds, etc., to achieve excellent Fluorescence performance, enhanced stability and longevity, effect of improved chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Its preparation method comprises the following steps:
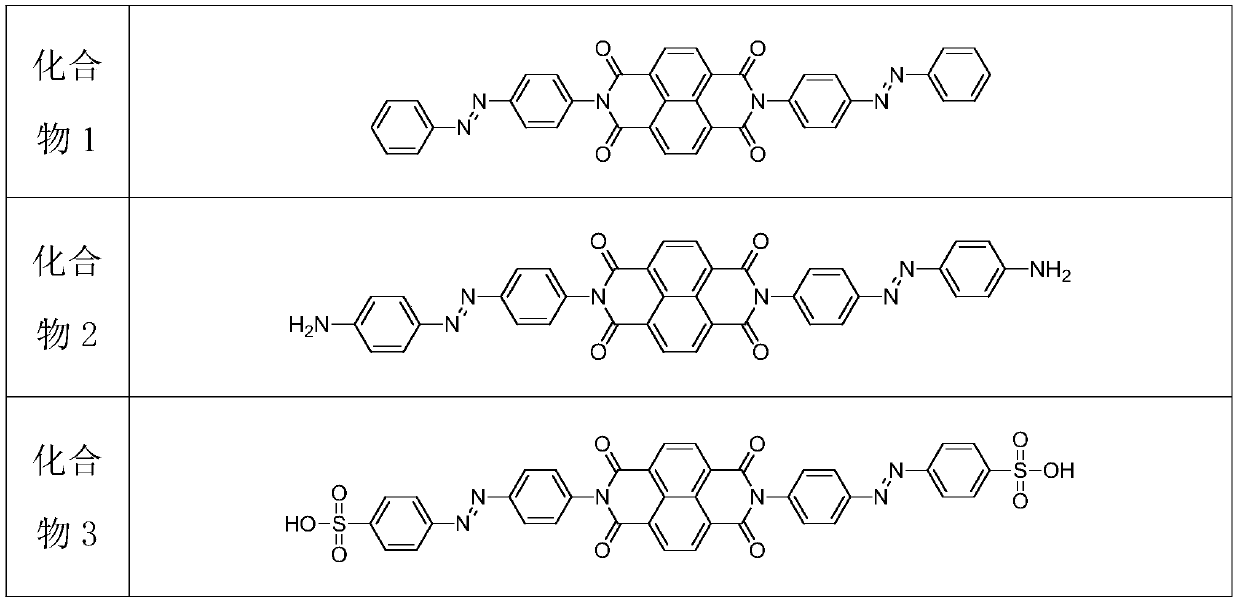
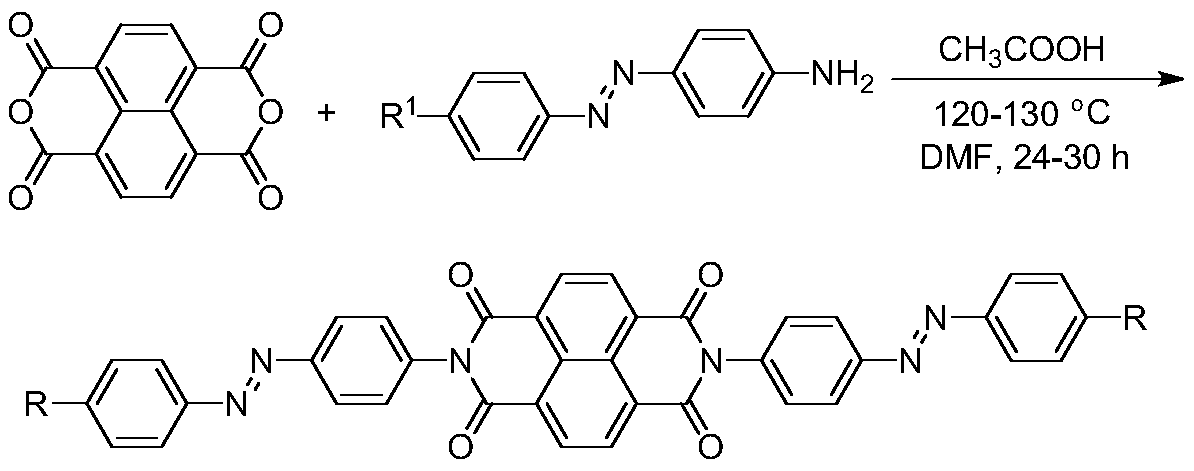
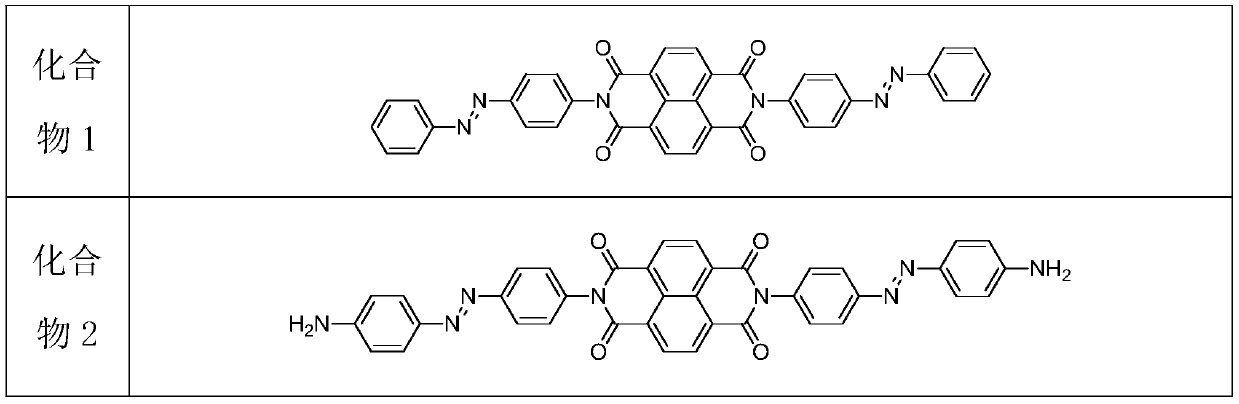
[0033] (1) Add 200-800mL of anhydrous N,N-dimethylformamide into the hydrothermal automatic reaction kettle, then weigh 1,4,5,8-naphthalene tetracarboxylic anhydride and azobenzene compounds in sequence Add glacial acetic acid slowly to the reaction kettle, the molar ratio of the three substances is 1:2.4-2.8:0.1-0.15, raise the temperature of the reaction kettle to 120-130°C, stir and react at a constant speed for 24-30h, and the reaction passes TLC Thin-layer chromatography was used to observe the reaction process. After the reaction of 1,4,5,8-naphthalene tetracarboxylic anhydride was complete, the solution was cooled to room temperature, and an appropriate amount of distilled water and ethyl acetate were added for extraction, and the organic phase of ethyl acetate was extracted. 3-5 times, the ethyl acetate organic phase was distilled off under reduced pressure by a rotary evaporator to remove ethyl acetate, an...
Embodiment 1
[0040] (1) Preparation of naphthalene tetracarbonyl diimide azobenzene derivatives: add 400mL of anhydrous N, N-dimethylformamide to the hydrothermal automatic reaction kettle, and then weigh 1, 4, 5, 8 - Add naphthalene tetracarboxylic anhydride and azobenzene into the reactor, and then slowly add it into glacial acetic acid, the molar ratio of the three substances is 1:2.4:0.1, raise the temperature of the reactor to 120°C, and stir at a constant speed for 30 hours, The reaction was observed by TLC thin-layer chromatography. After the reaction of 1,4,5,8-naphthalene tetracarboxylic anhydride was complete, the solution was cooled to room temperature, and an appropriate amount of distilled water and ethyl acetate were added for extraction. The organic phase was extracted 5 times, and the ethyl acetate organic phase was distilled off under reduced pressure by a rotary evaporator to remove ethyl acetate, and then the concentrated mixture was separated by thin-layer chromatography...
Embodiment 2
[0045](1) Preparation of naphthalene tetracarbonyl diimide azobenzene derivatives: add 800mL of anhydrous N,N-dimethylformamide to the hydrothermal automatic reaction kettle, and then weigh 1,4,5,8 - Naphthalene tetracarboxylic anhydride and azobenzene compounds are added to the reactor, and then slowly added into glacial acetic acid, the molar ratio of the three substances is 1:2.5:0.11, the temperature of the reactor is raised to 130°C, and the reaction is stirred at a constant speed 30h, the reaction was observed by TLC thin-layer chromatography. After the reaction of 1,4,5,8-naphthalene tetracarboxylic anhydride was complete, the solution was cooled to room temperature, and an appropriate amount of distilled water and ethyl acetate were added for extraction. The ethyl acetate organic phase was extracted 4 times, and the ethyl acetate organic phase was distilled off under reduced pressure by a rotary evaporator to remove ethyl acetate, and then the concentrated mixture was s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solid content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com