Polypeptide derivative, nanofiber and application of nanofiber
A nanofiber, peptide derivative technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of complex synthesis process, unfavorable long-term storage, poor water solubility, etc., and achieve a simple preparation process. , The effect of improving the organization retention rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The polypeptide derivatives are synthesized by the known FMOC-short peptide solid-phase synthesis method. Specifically, the preparation method of the polypeptide derivative includes the following steps:
[0061] (1) The C-terminal of the Fmoc-amino acid is bound to the resin;
[0062] (2) removal of Fmoc protecting group, washing;
[0063] (3) The C-terminal of the next Fmoc-amino acid is coupled to the N-terminal of the amino acid or polypeptide on the resin, and washed;
[0064] (4) Repeat steps (2) to (3) until the last amino acid coupling is completed, remove the Fmoc protecting group, and wash;
[0065] (5) X-OH is coupled and washed with the N-terminal of the polypeptide on the resin;
[0066] (6) The polypeptide derivative is excised from the resin to obtain a crude product;
[0067] (7) Use high performance liquid chromatography to purify the crude product.
[0068] According to the second aspect of the present invention, the present invention provides nano...
preparation Embodiment 1
[0094] Synthesis and Preparation of Peptide Derivative Nap-FFGSSSR and Its Nanofibers
[0095] (1) Solid phase synthesis of Nap-FFGSSSR
[0096] Specific steps are as follows:
[0097] 1) Weigh 0.5mmol 2-Cl-Trt resin in a solid-phase synthesizer, add 10mL of anhydrous dichloromethane (hereinafter referred to as DCM), place on a shaker and shake for 5min to fully swell the 2-Cl-Trt resin ;
[0098] 2) Remove the DCM from the solid-phase synthesizer equipped with 2-Cl-Trt resin with ear washing ball;
[0099] 3) Dissolve 0.75 mmol of Fmoc-Arg in 10 mL of anhydrous DCM, add 0.75 mmol of DIPEA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIPEA, and react at room temperature for 1 h;
[0100] 4) Sealing: Remove the reaction liquid in the solid-phase synthesizer with ear washing balls, then wash with 10 mL of anhydrous DCM, 1 min each time, and wash 5 times in total. Add the prepared volume ratio of anhydrous DCM: DIPEA: methanol = 17:1:2 solut...
Embodiment 1
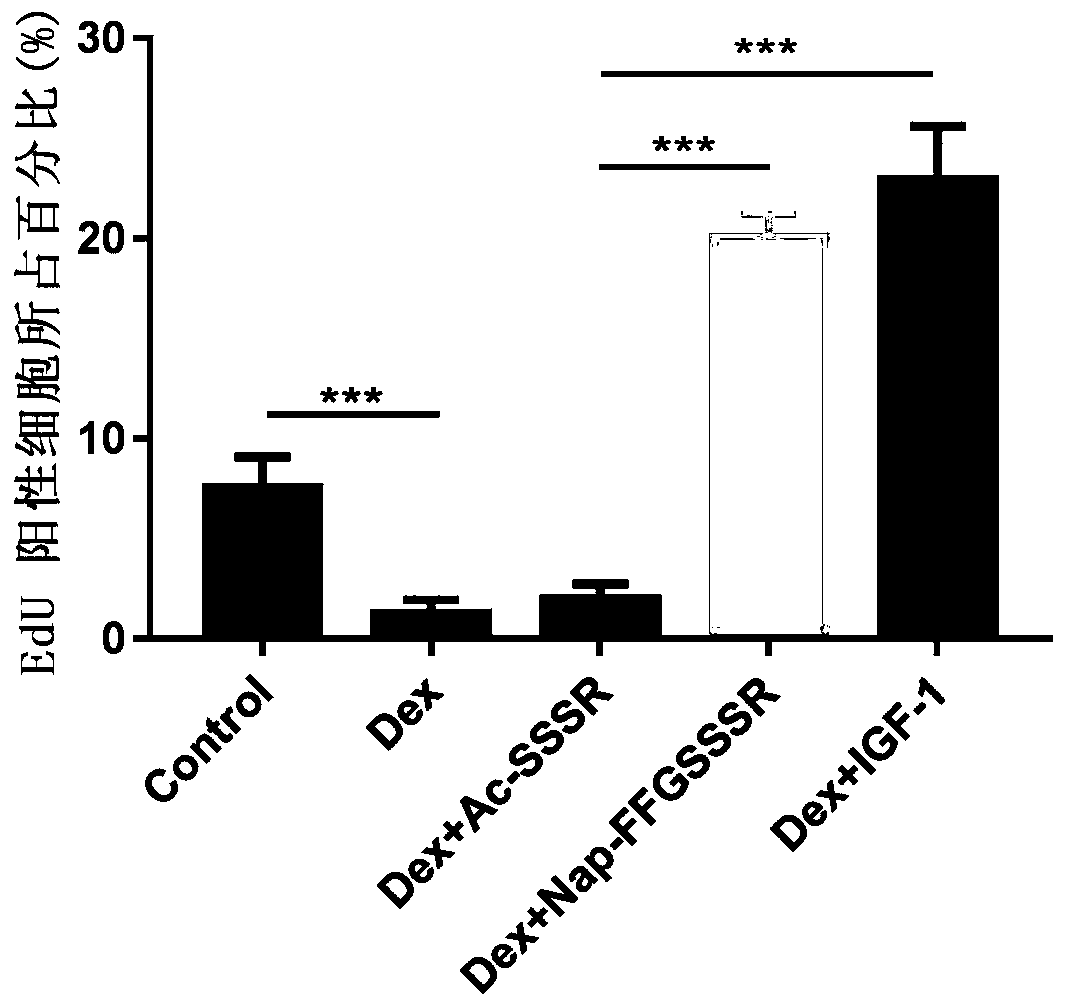
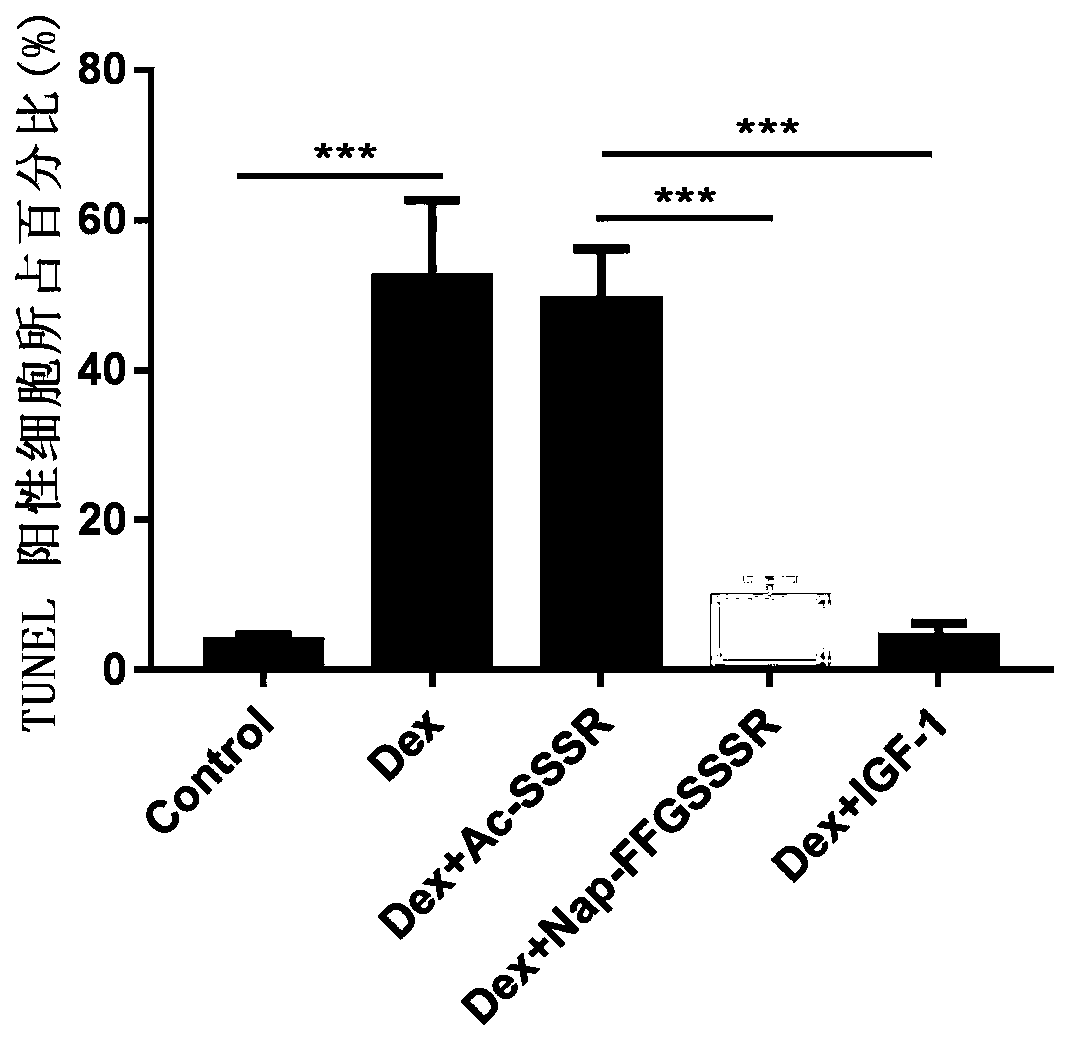
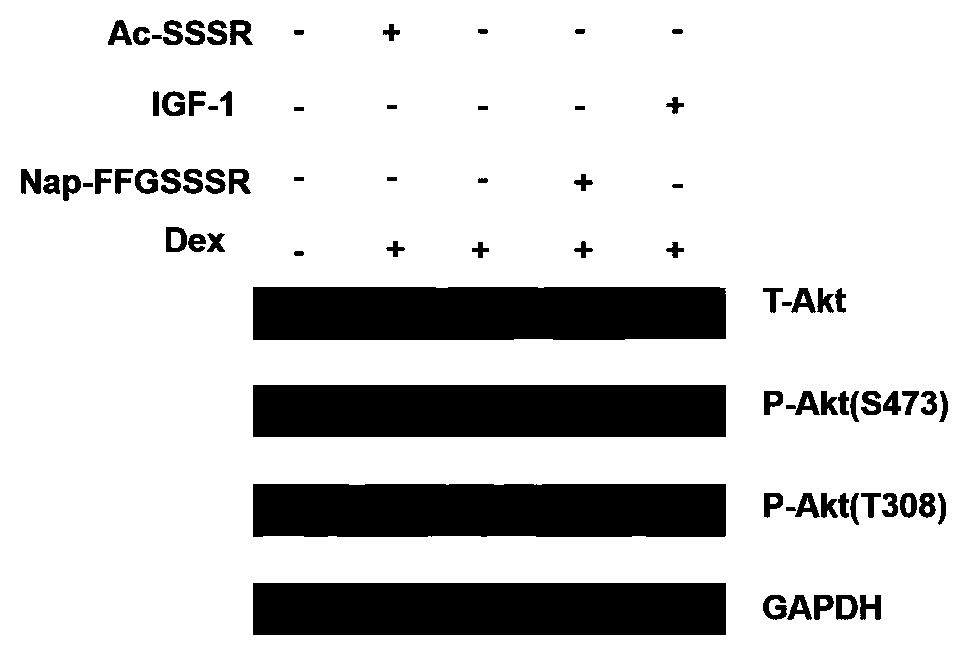
[0116] Activity Test of Hydrogel of Peptide Derivative Nap-FFGSSSR at the Cell Level
[0117] (1) Promoting the proliferation of mouse myoblasts (C2C12)
[0118] 1) Raise the temperature of the water bath to 37°C in advance, put the medium, serum, etc. into the water bath to preheat, and at the same time turn on the ultra-clean bench UV lamp for half an hour;
[0119] 2) Take out the frozen mouse myoblast C2C12 cells from the liquid nitrogen tank, quickly place them in a water bath at 37°C to thaw the cells, and then quickly transfer them to an ultra-clean bench for the following operations: transfer the cell-containing solution with a pipette Carefully transfer the liquid container to a centrifuge tube containing the medium, centrifuge for 3 minutes, remove the supernatant, resuspend with DMEM medium containing 10% fetal bovine serum, transfer to a petri dish, and then place it in a 37°C incubator to cultivate;
[0120] 3) Observe the state of the cells the next day. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com