Preparation method of trifluoroethyl compounds
A technology for trifluoroethyl compounds, applied in the field of preparation of trifluoroethyl compounds, can solve problems such as limited types of substrates and functional groups, and achieve the effects of easy reaction scale, simple operation and enlarged reaction scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
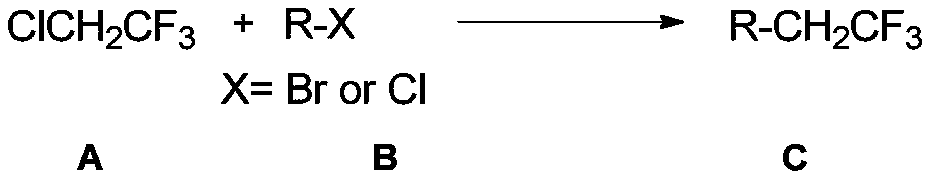
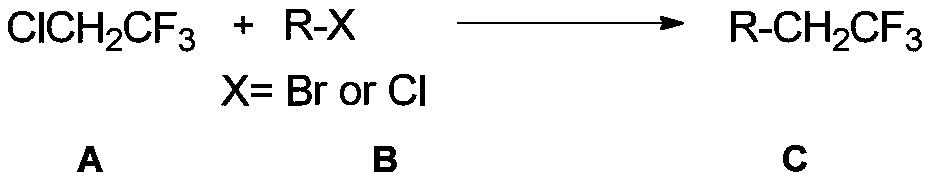
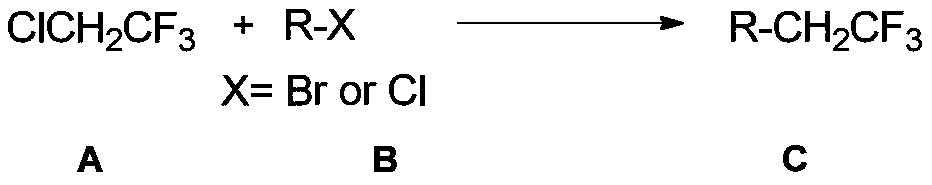
[0049] The coupling reaction of 2-chloro-1,1,1-trifluoroethane and (heterocyclic aryl)aryl bromide is a general preparation method of trifluoroethyl compounds.
[0050] Add anhydrous magnesium chloride (0.4mmol, 38.2mg, 1.0eq), nickel bromide (0.04mmol, 8.8mg, 0.1eq), ligand 4, 4'-di-tert-butyl-2,2'-bipyridine (0.04mmol, 10.8mg, 0.1 equivalent), bromobenzene or its derivatives (0.6mmol, 1.5 equivalent), then added DMA (4mL) and 2-chloro - 1,1,1-Trifluoroethane in DMA (0.4 mmol, 1.0 equiv). If the reaction is on a 0.2 mmol scale, the amounts of all the above reagents are halved.
[0051] The reaction mixture was heated to 80°C and stirred for 12 hours (or several times at other temperatures). It was then cooled to room temperature, diluted with 100 mL of ethyl acetate, washed with water and an appropriate amount of saturated saline in sequence, and then the organic phase was collected and dried over anhydrous sodium sulfate and concentrated under reduced pressure. After conce...
preparation example 2
[0053] The coupling reaction of 2-chloro-1,1,1-trifluoroethane and (heterocyclic aryl)aryl chloride is a general preparation method of trifluoroethyl compounds.
[0054] Add anhydrous magnesium chloride (0.6mmol, 57.2mg, 1.5eq), nickel bromide (0.04mmol, 8.8mg, 0.1eq), ligand 4, 4'-di-tert-butyl-2,2'-bipyridine (0.04mmol, 10.8mg, 0.1 equivalent), chlorobenzene or its derivatives (0.8mmol, 1.5 equivalent), then add DMA (4mL) and 2-chloro - 1,1,1-Trifluoroethane in DMA (0.4 mmol, 1.0 equiv). If the reaction is on a 0.2 mmol scale, the amounts of all the above reagents are halved.
[0055] The reaction mixture was heated to 80°C and stirred for 12 hours (or several times at other temperatures). It was then cooled to room temperature, diluted with 100 mL of ethyl acetate, washed with water and an appropriate amount of saturated saline in sequence, and then the organic phase was collected and dried over anhydrous sodium sulfate and concentrated under reduced pressure. After conce...
Embodiment 1
[0057] The trifluoroethyl compounds prepared in Example 1 are specifically
[0058] The reaction scale was 0.2 mmol and the product was a pale yellow oily liquid. 19F NMR (376MHz, CDCl3) δ-66.0 (t, J=10.5Hz, 3F). MS (GC-MS): 160.1. When X=Br, the yield was 73%; when X=Cl, the yield was 74%. The product has a low boiling point and the yield passes through 19 F NMR determination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com