Steady-state petunidin-3-O-glucoside derivative and preparation method thereof
A technology of petunidin and glycoside derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of poor stability of anthocyanins, and achieve good light and heat stability and cell inhibition rate increase, the effect of increasing the cell proliferation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the stabilized petunidin-3-O-glucoside derivative of the present invention comprises the following steps:
[0036] Step 1, destemming the mountain grapes, beating, and obtaining the mountain grape slurry;
[0037] Step 2, using a non-thermal extraction technique to extract the anthocyanins in the grape sap to obtain an anthocyanin solution;
[0038] Step 3, centrifuging the anthocyanin solution, taking the supernatant, concentrating under reduced pressure, eluting with a macroporous resin, combining the eluents, concentrating under reduced pressure, and freeze-drying to obtain a purified anthocyanin;
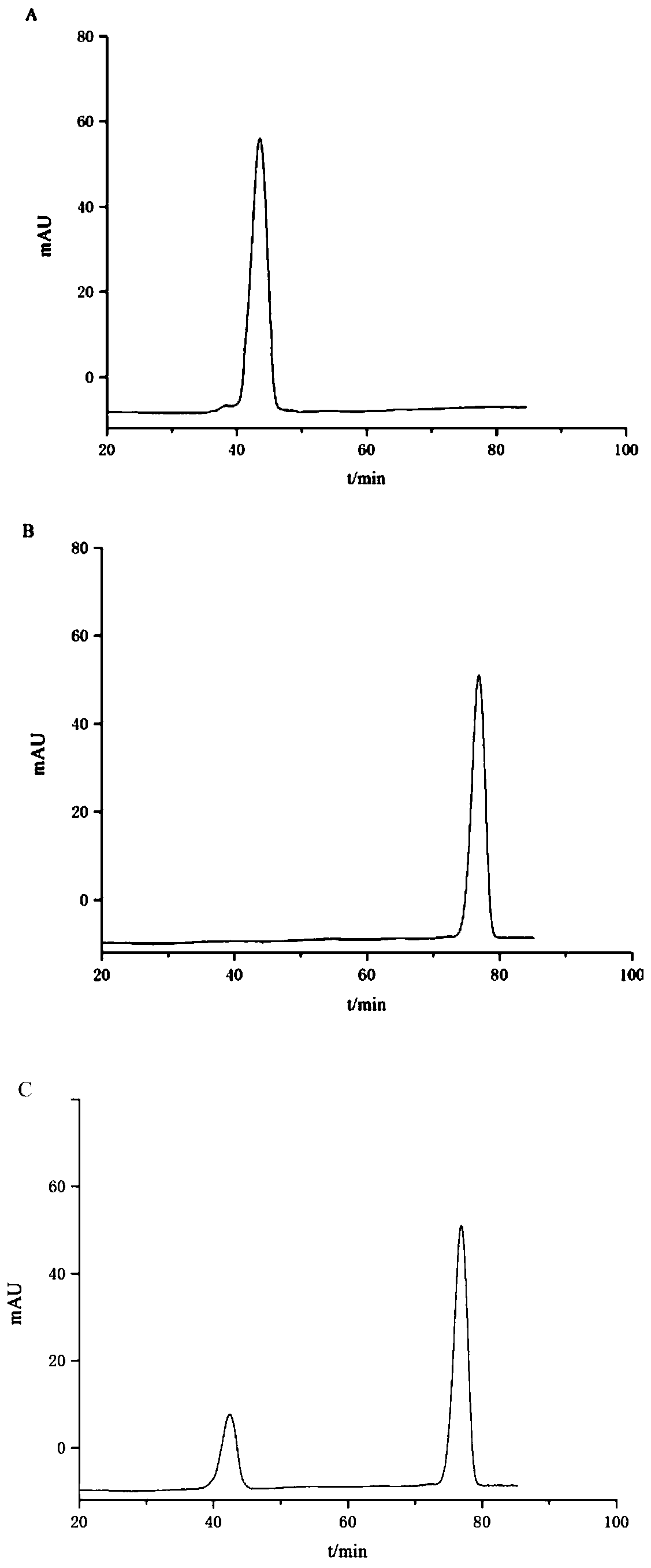
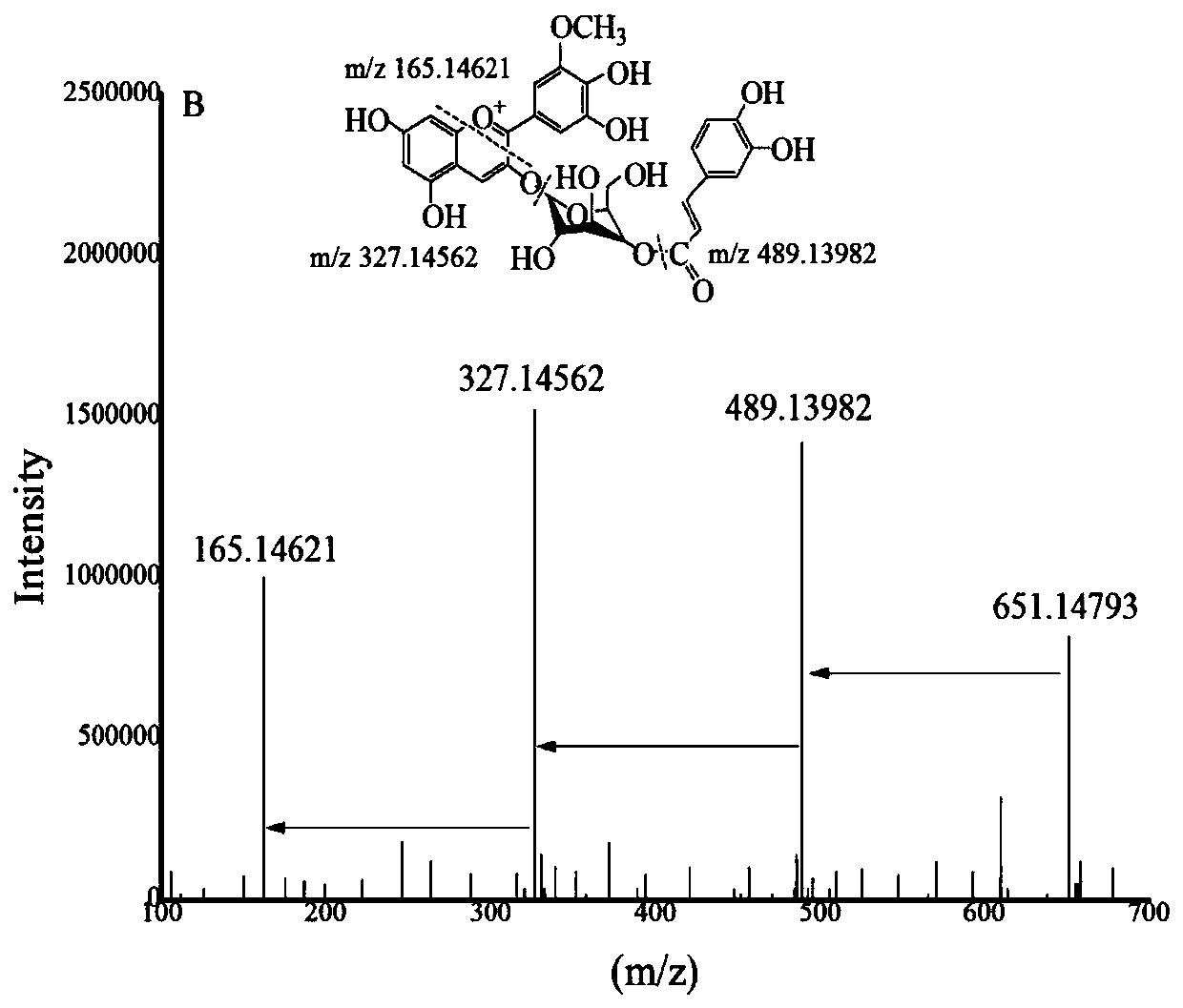
[0039] Step 4. Dissolve the purified anthocyanin in methanol solution containing 0.5-1% (v / v) HCl, separate by preparative liquid chromatography, collect the elution peaks, and freeze-dry to obtain Petunia-3-O- Glucoside;
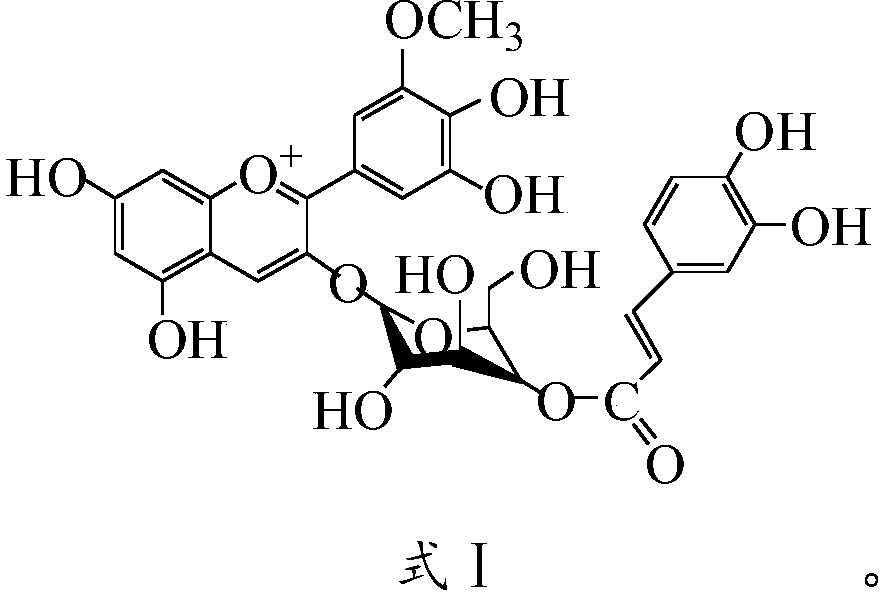
[0040] Step 5. Dissolve caffeic acid and petunienin-3-O-glucoside in an organic solvent in a mass ratio of 1:(3-5), mix we...
Embodiment 1
[0050] Step 1, destemming the mountain grapes, beating, and obtaining the mountain grape slurry;
[0051] Step 2, using a high-voltage pulsed electric field to extract the anthocyanins in the vine grape slurry to obtain an anthocyanin solution; wherein, the extraction solvent is 0.1% hydrochloric acid-75% ethanol solution, the liquid-to-material ratio is 9:1w / w, and the electric field strength is 15Kv / cm , pulse number 4;
[0052] Step 3, centrifuge the anthocyanin solution, take the supernatant, concentrate under reduced pressure (to a relative density of 1.1, 50°C), elute with a macroporous resin, combine the eluents, and concentrate under reduced pressure (to a relative density of 1.3 , 50°C), freeze-dried to obtain purified anthocyanins; wherein, the conditions for preparative liquid chromatography separation were: chromatographic column: SunFire Prep C 18 Column, size 19mm×50mm, 5μm; detection wavelength 530nm; column temperature 25°C; flow rate 5mL / min; 15%B; 5~8min, 1...
Embodiment 2
[0056] Step 1, destemming the mountain grapes, beating, and obtaining the mountain grape slurry;
[0057] Step 2, using a high-voltage pulsed electric field to extract the anthocyanins in the vine grape slurry to obtain an anthocyanin solution; wherein, the extraction solvent is 0.1% hydrochloric acid-75% ethanol solution, the liquid-to-material ratio is 9:1w / w, and the electric field strength is 15Kv / cm , pulse number 4;
[0058] Step 3, centrifuge the anthocyanin solution, take the supernatant, concentrate under reduced pressure (to a relative density of 1.1, 50°C), elute with a macroporous resin, combine the eluents, and concentrate under reduced pressure (to a relative density of 1.2 , 50°C), freeze-dried to obtain purified anthocyanins;
[0059] Step 4. Dissolve the purified anthocyanin in methanol solution containing 0.5-1% (v / v) HCl, separate by preparative liquid chromatography, collect the elution peaks, and freeze-dry to obtain Petunia-3-O- Glucoside; Wherein, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com