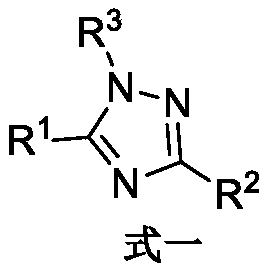
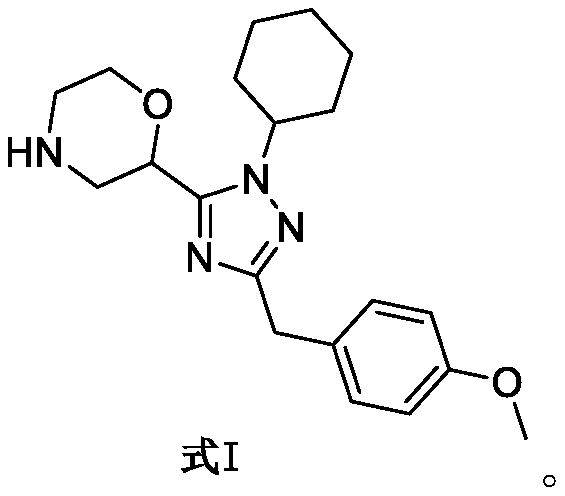
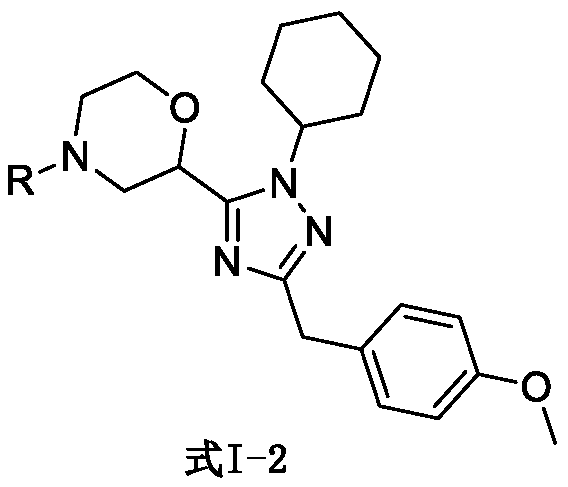
SET8 lysine methyltransferase inhibitor, intermediate, preparation method and applications thereof
A technology for lysine methyl and inhibitors, applied in the field of preparation of SET8 lysine methyltransferase inhibitors and intermediates thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of tert-butyl 2-((2-(4-methoxyphenyl)thioacetyl)carbamoyl)morpholine-4-carboxylate (formula II)
[0046]
[0047] Starting materials 4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid (1.0 mmol, 231 mg), 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyl Urea hexafluorophosphate (HATU, 1.1mmol, 418mg) was dissolved in 4mL of dry dichloromethane, under nitrogen protection, after stirring at room temperature for 20 minutes, the raw material 2-(4-methoxyphenyl ) Thioacetamide (1.1mmol, 199mg), after continuing to stir for 1 hour, N,N-diisopropylethylamine (DIEA, 1.1mmol, 142mg) was added dropwise into the above reaction system, and continued under nitrogen protection at room temperature The reaction was stirred for 2 days. After the reaction solution was diluted with 50 mL of ethyl acetate, it was washed with 20 mL of water and saturated sodium chloride solution successively, dried over anhydrous sodium sulfate, and after concentrating the react...
Embodiment 2
[0048] Example 2 Preparation of 2-(1-cyclohexyl-3-(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine-4-carboxylic acid tert-butyl ester ( Formula III)
[0049]
[0050] Formula II (0.16mmol, 63mg), cyclohexylhydrazine hydrochloride (0.2mmol, 30mg), sodium acetate (0.36mmol, 30mg) were successively dissolved in a mixed solvent of 1mL acetic acid and 1mL 1,4-dioxane Finally, seal it, and then react under heating at 80°C until the reaction of II is complete. After the reaction solution was diluted with 30mL ethyl acetate, washed successively with 20mL saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, after concentrating the reaction solution, column chromatography separated [V (petroleum ether): V( Ethyl acetate)=3:1-1:1], 40 mg of light yellow oily substance III was obtained, and the yield was 55%.
Embodiment 3
[0051] Example 3 Preparation of 2-(1-cyclohexyl-3-(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine (Formula I)
[0052] Formula III (0.09mmol, 41mg), anisole (0.1mmol, 11mg), 0.5mL of hydrogen chloride in 1,4-dioxane solution (4M) were successively dissolved in 3mL of dichloromethane, and sealed for 3 days , to complete the reaction of III. The reaction solution was evaporated to dryness to obtain a yellow oil, which was diluted with 30 mL of ethyl acetate, washed with 20 mL of saturated sodium carbonate solution, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography [V(ammonia di Chloromethane solution): V (methanol) = 25:1-20:1] to obtain 26 mg of yellow oil I, with a yield of 81%.
[0053] 1 H NMR (400MHz, CDCl 3 ):δ7.25-7.23(m,2H),6.82-6.80(m,2H),4.67-4.64(m,2H),4.28-4.21(m,2H),3.98(s,2H),3.85-3.79 (m,1H),3.77(s,3H),3.73-3.67(m,1H),3.31-3.25(m,1H),3.19-3.15(m,1H),3.01-2.87(m,2H),1.98 -2.85(m,6H),1.73-1.70(m,1H),1.40-1.26(m,4H). 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com