Application of p.P476S mutation of RBPJL gene as PD-1 antibody medication guiding marker
A technology of p.p476s and PD-1, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems of lack of treatment response and treatment resistance mechanism, achieve good application prospects and value, and improve treatment effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
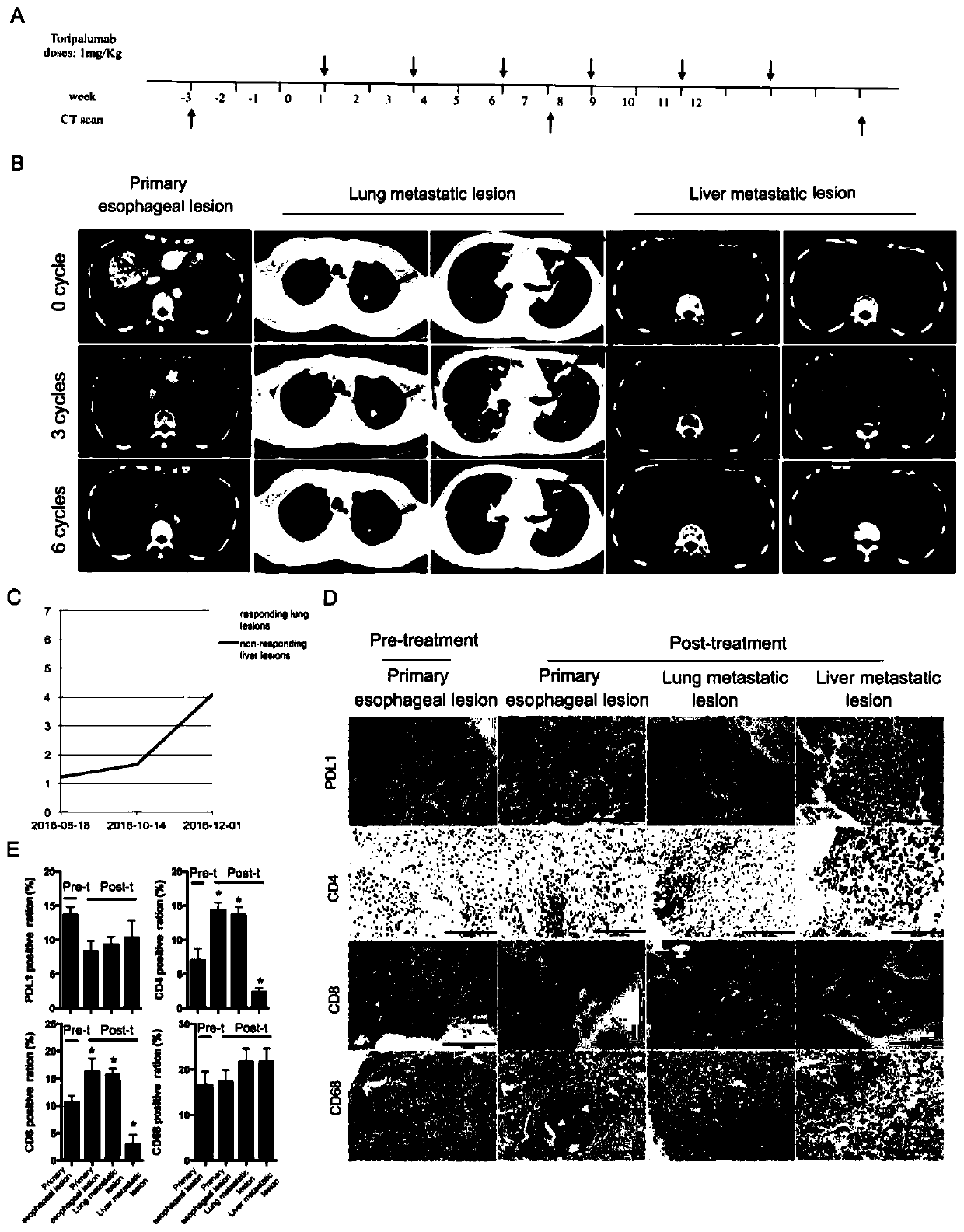
[0025] Embodiment 1 clinical patient's toripalimab treatment situation
[0026] 1. Patient and Treatment
[0027] The patient was initially diagnosed as stage IV moderately differentiated esophageal cancer in the middle and lower part of the esophagus. On March 11, 2016 and April 06, 2016, platinum and paclitaxel chemotherapy regimens were used for 2 courses, and the curative effect was evaluated as PD (tumor progression). From May 7, 2016 to August 8, 2016, 7 courses of irinotecan chemotherapy were given as second-line chemotherapy, among which the curative effect was evaluated as SD after 3 courses of treatment, and PD (tumor progression) after 7 courses of treatment. Afterwards, the patient underwent a Phase I clinical trial (NCT02857166) of 6 cycles of toripalimab treatment (1mg / kg, q2w) in the Cancer Prevention and Control Center of Sun Yat-sen University.
[0028] The flow chart of the patient's PD1 treatment process is shown in figure 1 a.
[0029] 2. CT scan
[003...
Embodiment 2
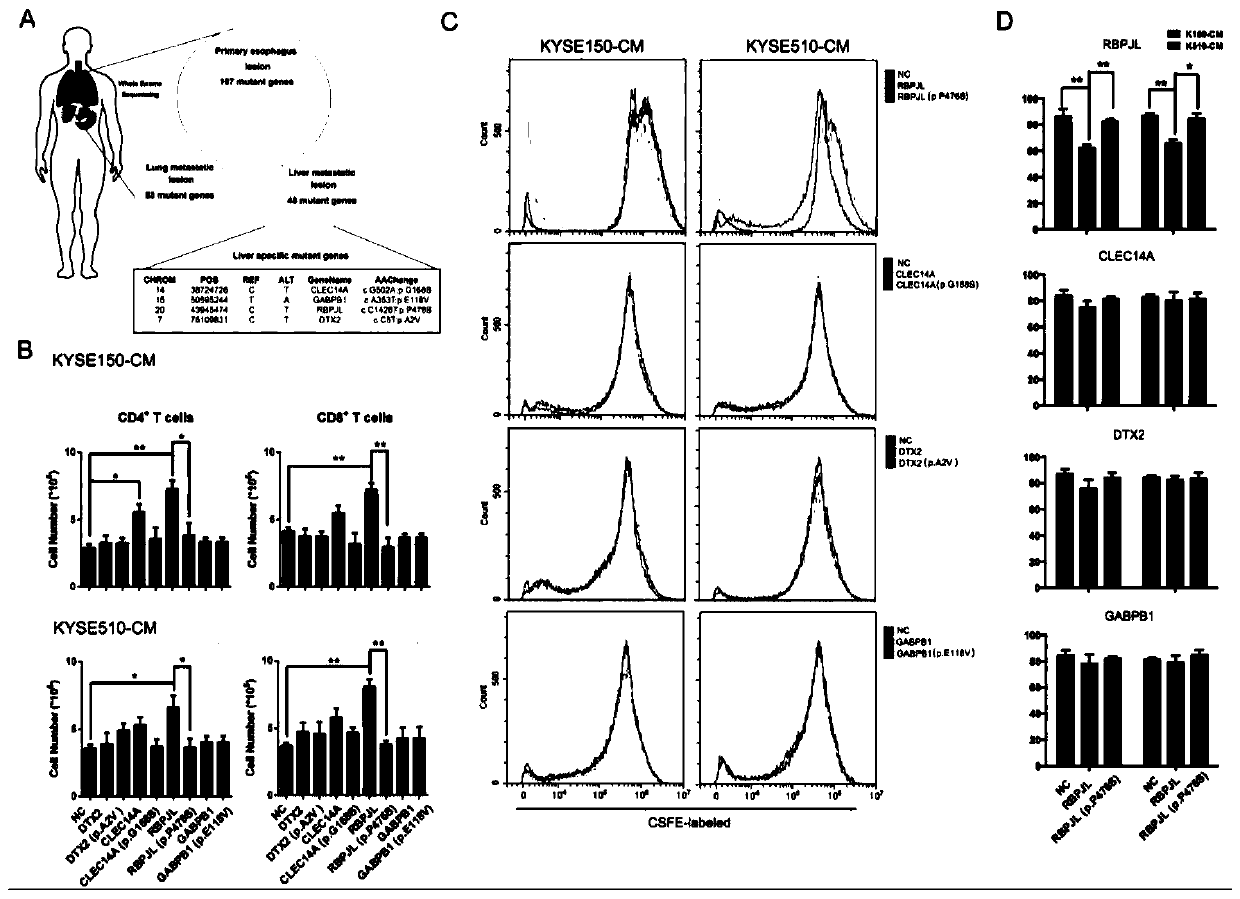
[0051] Example 2 Screening of Liver Metastases Specific Mutation Genes
[0052] 1. Exome sequencing of primary and metastatic tissues of clinical patients
[0053] 1. Experimental method
[0054] To explore the genomic signatures of resistance to toripalimab in liver metastatic tissues from patients, DNA was extracted from primary and metastatic tissues after treatment and whole-exome sequencing was performed.
[0055] Total DNA was extracted from post-treatment plasma and primary and metastatic tumor biopsies, and then whole-exome sequencing was performed using Illumina Hiseq 2000. In the process of data processing, BWA-MEM. technology is used to compare and calibrate the human genome (HG19) data in UCSC at the same time, so as to identify high-quality genetic data. picard (v1.84; http: / / broadistitute.github.io / picard / ) was used to label and classify duplicate reads of data during PCR, followed by the genome analysis toolkit (gatk4, http: / / www .broadistitute.org / gatk) for ...
Embodiment 3
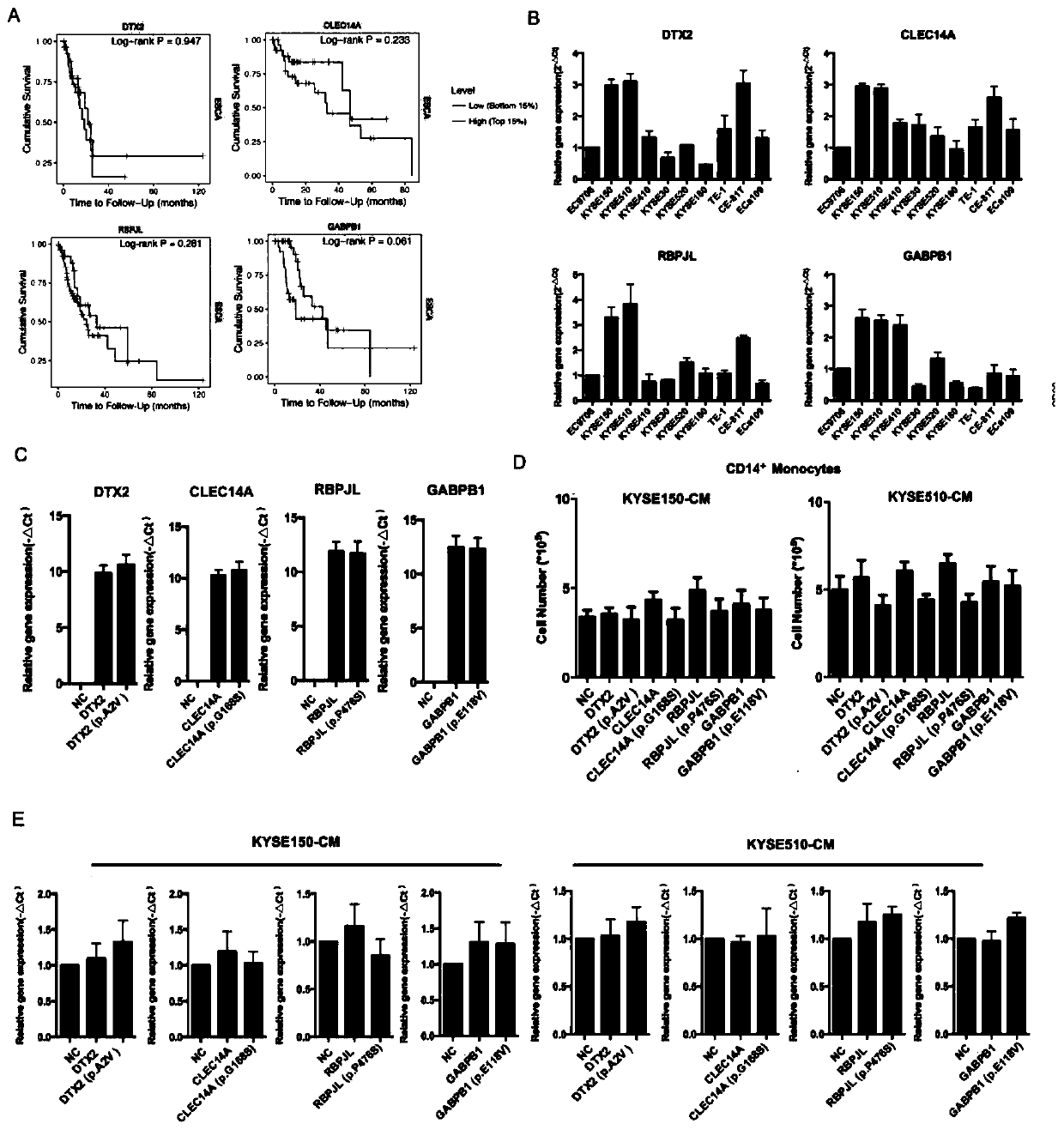
[0112] Example 3 RBPJL mutation affects T cell immune regulation ability
[0113] 1. Experimental method
[0114] Further studies were performed to detect the effects of different gene treatment groups of CMs on the differentiation of CD4+T cells. The specific experimental steps are as follows:
[0115] (1) After separating PBMCs by Ficoll-Paque Plus gradient centrifugation, CD3 magnetic beads were used to further purify T cells from PBMCs by positive selection and culture them in serum-free ImmunoCult-XF T Cell ExpMedium, and add CD3 / CD28 and IL- 2 stimulate the activation of T cells;
[0116] (2) Add the conditioned medium overexpressing each gene to the T cells and culture for 2 days; after the cell treatment, gently aspirate the medium, wash twice with pre-cooled PBS, and collect the cells;
[0117] (3) 1000rpm, centrifuge at room temperature for 5min, wash 3 times with preheated PBS, add diluted flow cytometry antibodies (CD4-PE, T-bet-PerCP / Cyanine5.5 and GATA-3-Alexa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com