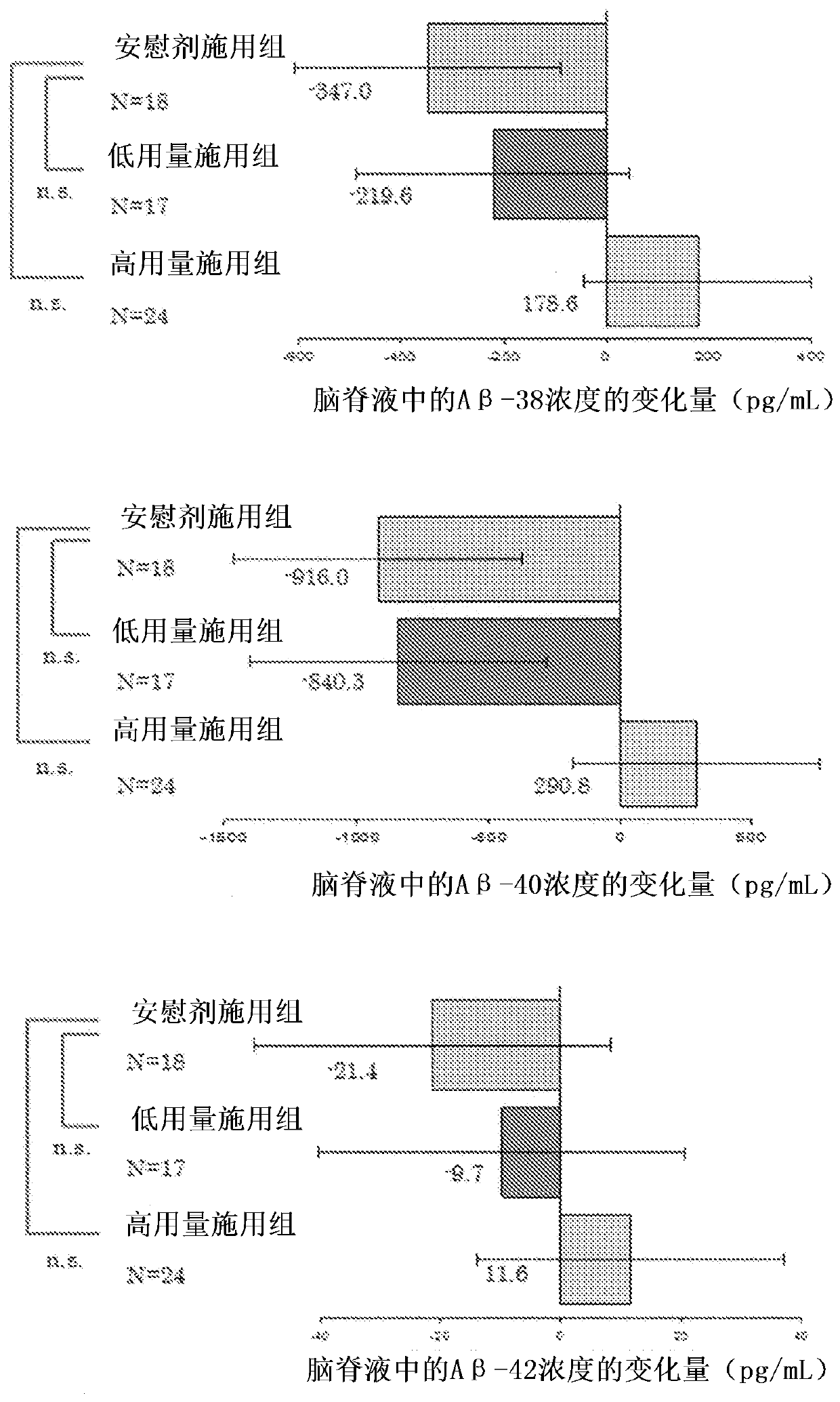
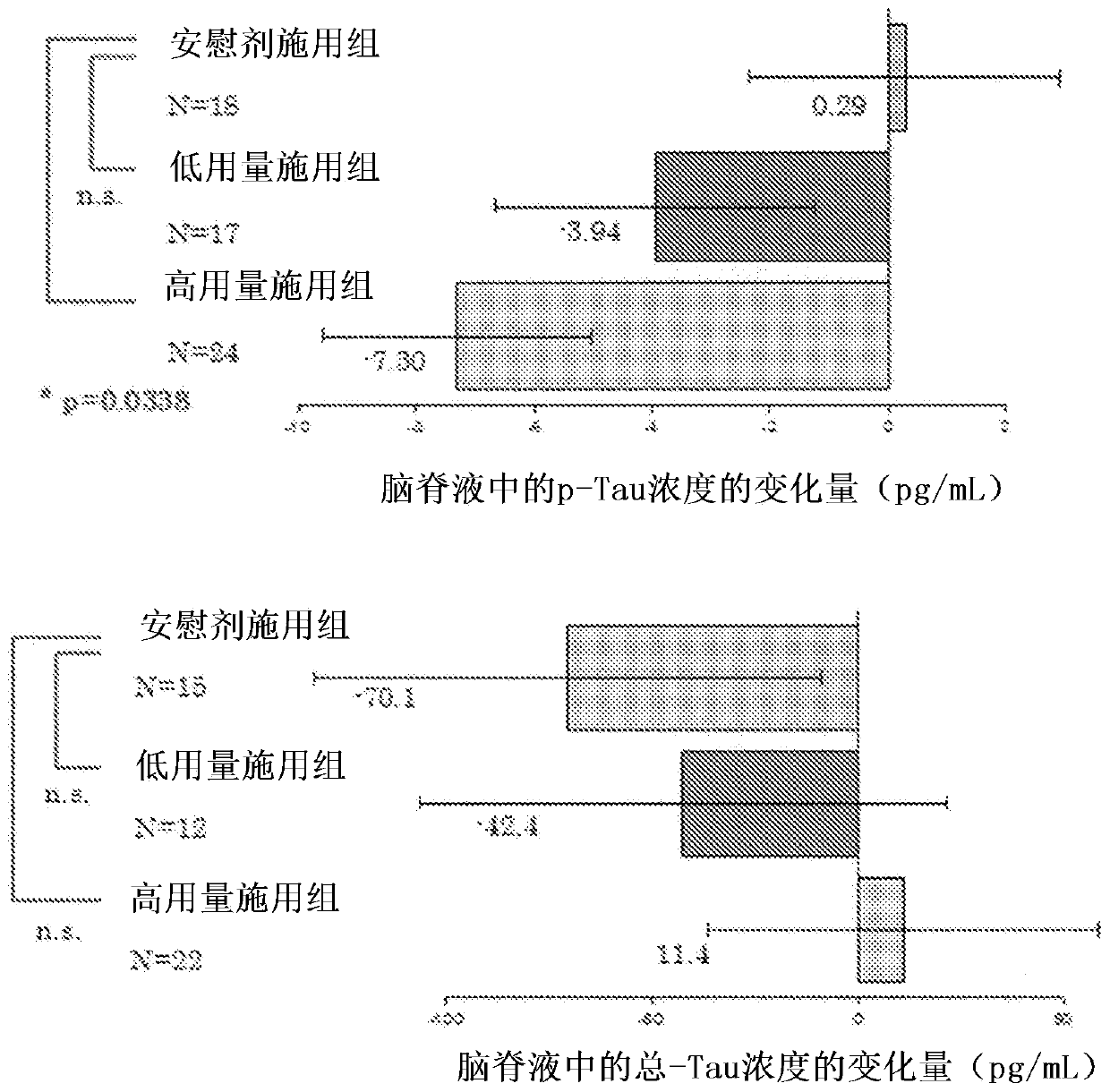
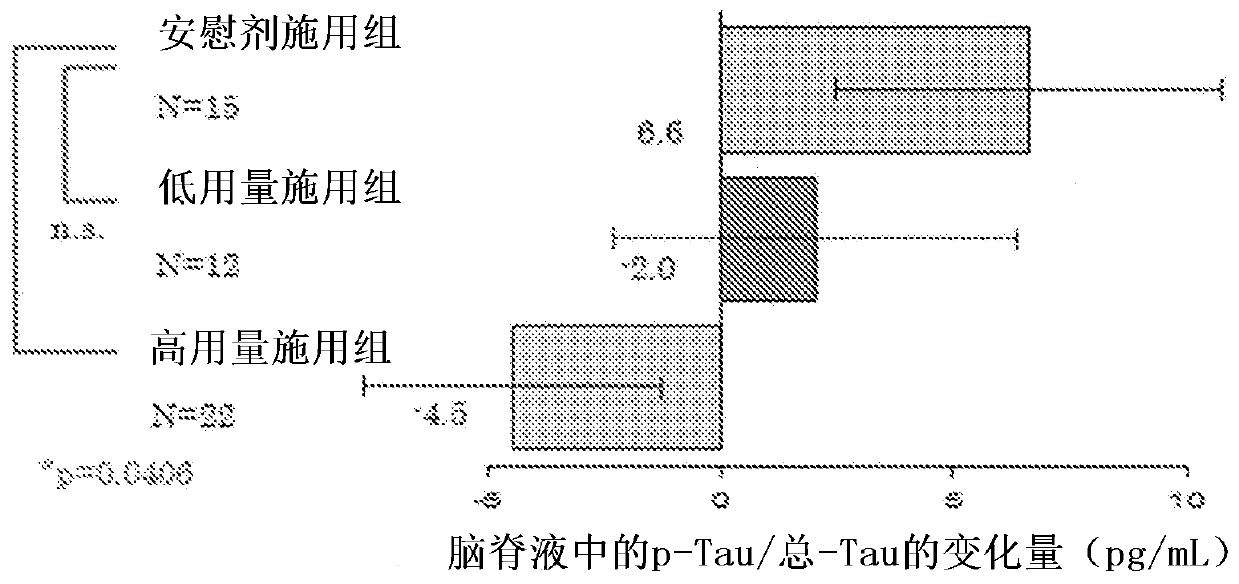
Agent for preventing or treating tauopathy
A technology of protein disease and therapeutic agent, applied in the field of prevention or treatment of protein tauopathies, can solve problems such as poor cognitive function and cognitive function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0128] 0.9726 g of magnesium stearate (magnesium stearate, Merck) was added to 174.03 g of the maleate salt of compound A, and mixed for 30 minutes. This mixed powder was compressed and molded with a dry granulator (TF-LABO (roller pressure 3 MPa), Freund Industries), and the molded solid was sized. To 60.0 g of the obtained sized powder, 49.51 g of lactose (Flowlac90, Meggle Japan), 16.50 g of crystalline cellulose (Ceolus PH302, Asahi Kasei chemicals) and croscarmellose sodium were added by sieving through a sieve with a mesh size of 850 μm. (primellose, DMV Japan) 6.67 g, mixed for 10 minutes. 0.6667 g of magnesium stearate was added to this mixed powder, and it mixed for 30 minutes. This mixed powder was compressed using a double arc pestle with a tablet diameter of 8.5 mm, and was compressed by a tablet press (HT-P18A, Tobacco Iron Works) at a compression pressure of about 12 kN to obtain a round tablet of 250 mg. shaped die. Utilize coating machine: DRC-200 (powrex) i...
preparation example 2
[0130] 60.90 g of mannitol (Parteck M200, Merck) and 3.60 g of croscarmellose sodium were added to 53.70 g of the maleate salt of compound A, and mixed for 10 minutes. 1.80 g of magnesium stearate was added to this mixed powder, and it mixed for 30 minutes. This mixed powder was tableted using a double arc pestle with a tablet diameter of 8.5 mm at about 10 kN to obtain 250 mg of round bare tablets. Coating agent (Opadry 03F44057, 00F440000 (hypromellose 2910: 71.5%, PEG 6000: 14.166%, talc: 7.167%, titanium oxide: 7.067%, iron oxide: 0.1%) was applied to the bare tablet, Japan Colorcon) was coated at a ratio of 8 mg per tablet, and a small amount of carnauba wax was added to obtain film-coated tablets.
preparation example 3
[0132] 11.11 g of magnesium stearate was added to 1988.89 g of the maleate salt of compound A, and mixed for 30 minutes. The mixed powder is compression-molded with a dry granulator, and the shaped solid is sized. Add mannitol 26.21g, ethyl cellulose (ETHOCEL100FP premium, Dow Chemical) 7.50g, crystalline cellulose (ceolus KG-1000, Asahi Kasei Chemicals) 3.75g, crospovidone ( Kollidon CL-SF, BASF) 3.75 g and croscarmellose sodium 0.75 g were mixed for 30 minutes. 0.90 g of magnesium stearate was added to the mixed powder, and mixed for 5 minutes. This mixed powder was tableted using a double-arc pestle with a tablet diameter of 8.5 mm at a tableting pressure of about 7 kN to obtain 315 mg of round bare tablets. The bare tablets were coated with a coating agent at a rate of 9 mg per tablet, and then a small amount of carnauba wax was added to obtain film-coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com