Fluorescent small-molecular probes based on single benzene ring and synthesis method thereof
A technology of small molecule probes and synthesis methods, applied in the field of fluorescent small molecule probes and their synthesis, can solve the problems of no fluorescence, destroying fluorescence behavior, etc., and achieve a wide range of solvent applications, good photochemical stability, and high fluorescence quantum yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]Weigh ethyl 4-bromobenzoate, RuPhos-G3-Palladacycle, 2-dicyclohexylphosphine-2′,6′-diisopropoxy-1,1′-biphenyl (RuPhos) and Place cesium carbonate in a two-necked bottle, connect it to a double-row tube system to ensure a nitrogen atmosphere, dissolve the reactant with dry 1,4-dioxane, inject azetidine, and heat to reflux for 12- After 18 hours, the reaction was filtered, concentrated, and column separation was performed with dichloromethane:petroleum ether=1:5 to obtain the compound 4-azetidinylbenzoic acid ethyl ester. Among them, the molar ratio of ethyl 4-bromobenzoate, RuPhos-G3-Palladacycle, RuPhos, cesium carbonate and azetidine is 1:0.1:0.1:1:1.5.
[0077] The proton nuclear magnetic spectrum data of compound 1 (4-azetidinylbenzoic acid ethyl ester) is: 1 H NMR (600MHz, C 6 D. 6 )δ(ppm)8.27(m,2H),6.12(d,J=8.4Hz,2H),4.23(m,2H),3.30(m,4H),1.64(m,2H),1.09(t,J =7.2Hz, 3H).
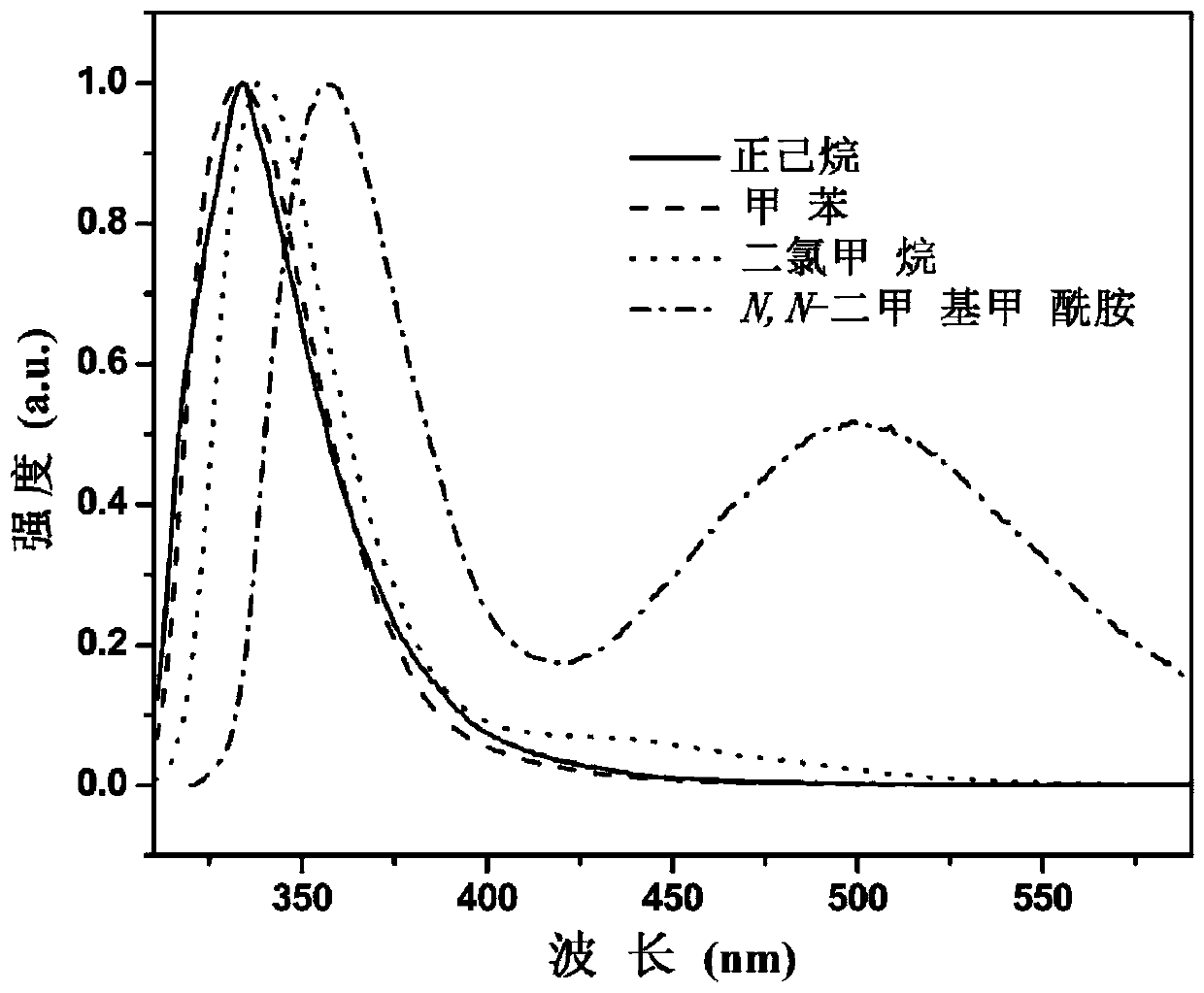
[0078] see figure 1 , figure 1 Fluorescence emission spectra of the compound 4-azetidin...
Embodiment 2
[0080] Weigh ethyl 2-bromobenzoate, RuPhos-G3-Palladacycle, 2-dicyclohexylphosphine-2′,6′-diisopropoxy-1,1′-biphenyl (RuPhos) and Place cesium carbonate in a two-necked bottle, connect it to a double-row tube system to ensure a nitrogen atmosphere, dissolve the reactant with dry 1,4-dioxane, inject azetidine, and heat to reflux for 12- After 18 hours, the reaction was filtered, concentrated, and column separation was performed with dichloromethane:petroleum ether=1:3 to obtain the syrupy compound ethyl 2-azetidinylbenzoate. Among them, the molar ratio of ethyl 2-bromobenzoate, RuPhos-G3-Palladacycle, RuPhos, cesium carbonate and azetidine is 1:0.1:0.1:1:1.5.
[0081] The proton nuclear magnetic spectrum data of compound 2 (2-azetidinylbenzoic acid ethyl ester) are: 1 H NMR (600MHz, C 6 D. 6 )δ (ppm) 7.86 (d, J = 7.2Hz, 1H), 7.14 (m, 1H), 6.64 (t, J = 7.8, 15.0Hz, 1H), 6.26 (m, 1H), 4.13 (q, J =6.6Hz, 2H), 3.61(m, 4H), 1.71(m, 2H), 1.06(t, J=6.6, 13.8Hz).
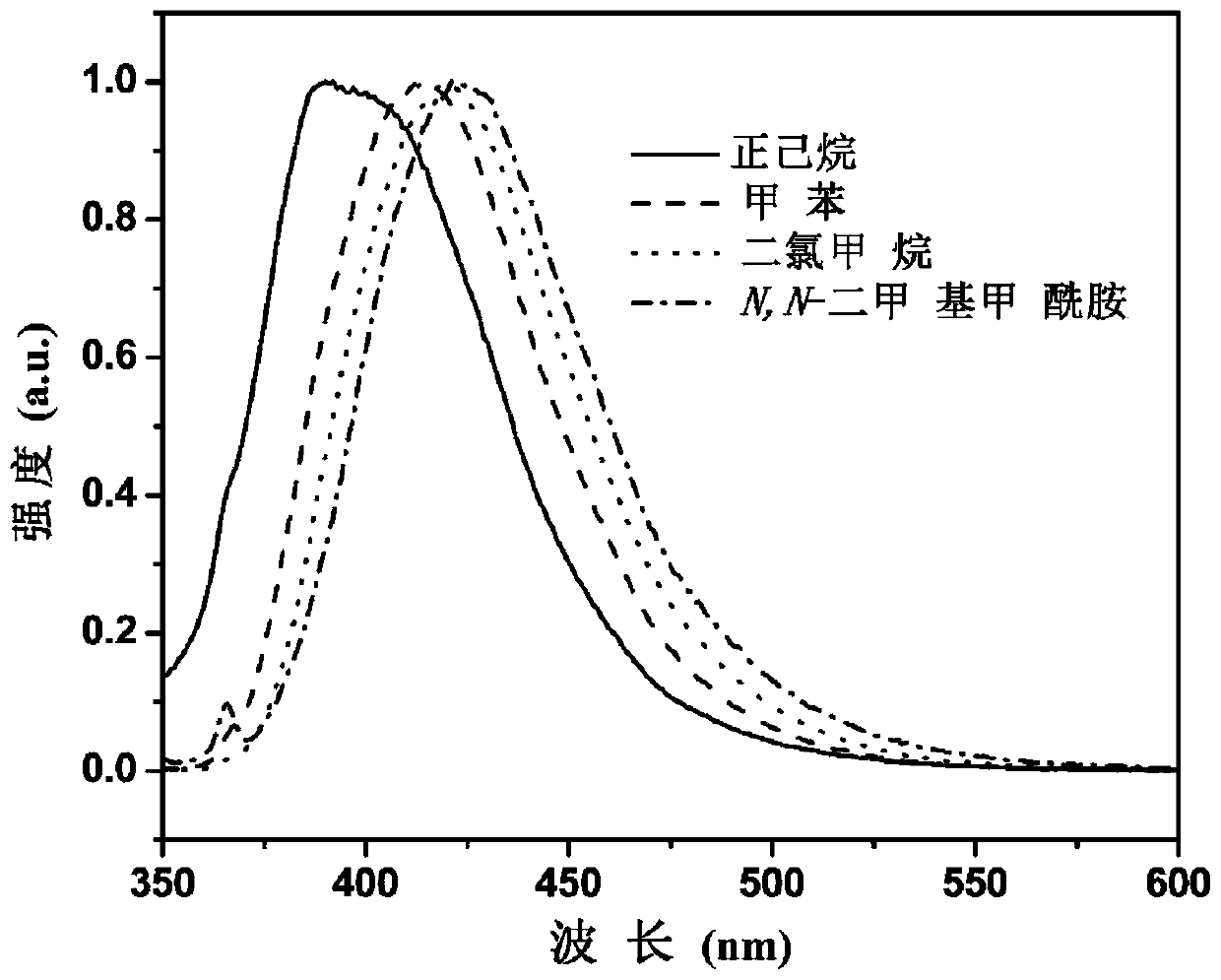
[0082] see fi...
Embodiment 3
[0084] Weigh 2,5-dibromoethyl terephthalate, RuPhos-G3-Palladacycle, 2-dicyclohexylphosphine-2′,6′-diisopropoxy-1,1′-linked Benzene (RuPhos) and cesium carbonate are placed in a two-necked bottle, connected to a double-row tube system to ensure a nitrogen atmosphere, dissolved with dry 1,4-dioxane, and then injected with azetidine, heated to reflux After 12 to 18 hours, after the reaction, filter and concentrate, and conduct column separation with dichloromethane:petroleum ether=1:1 to obtain ethyl 2-azetidinyl-5-bromoterephthalate. Among them, the molar ratio of 2,5-dibromoethyl terephthalate, RuPhos-G3-Palladacycle, RuPhos, cesium carbonate and azetidine is 1:0.1:0.1:1.5:2.
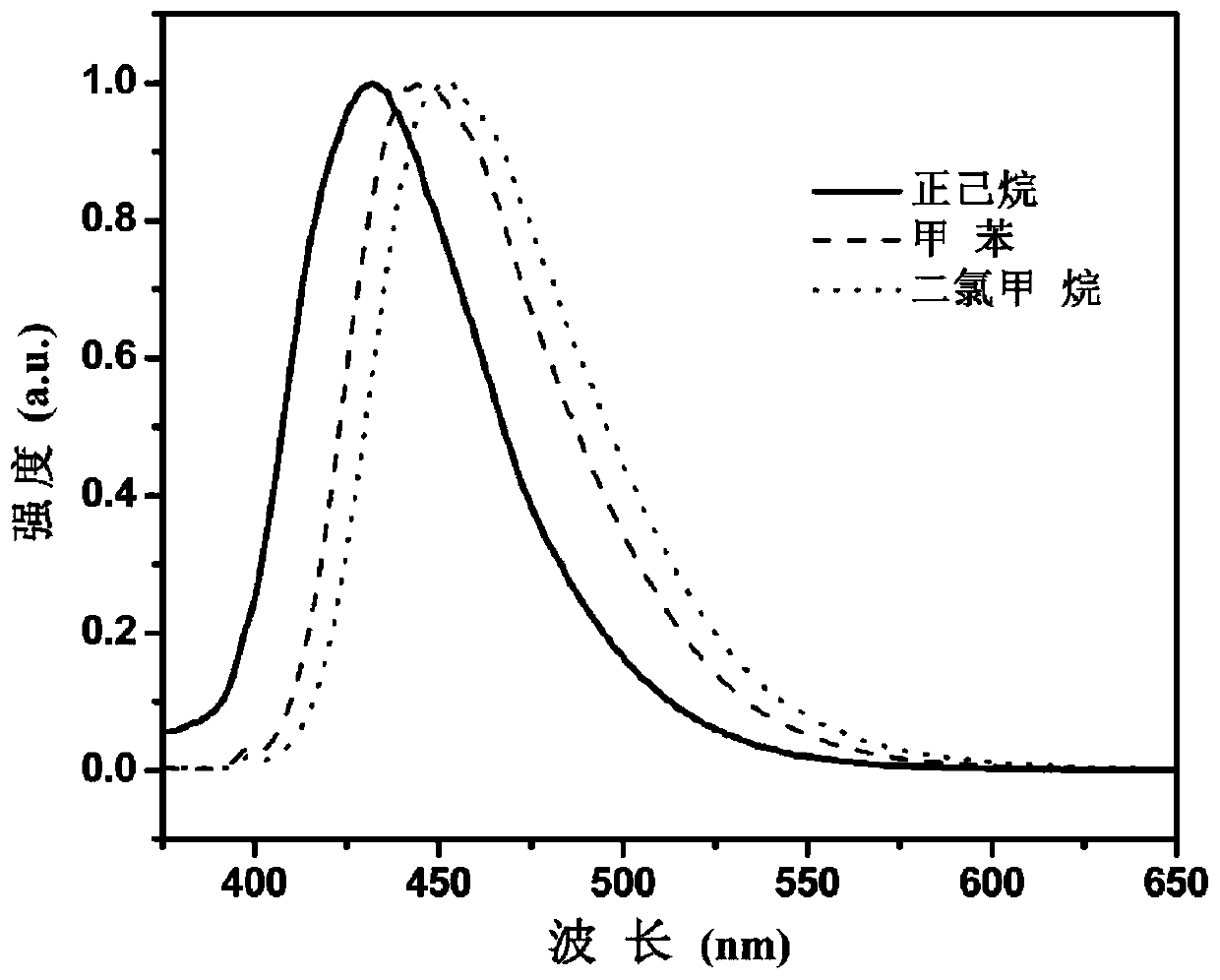
[0085] Under the protection of argon, weigh ethyl 2-azetidinyl-5-bromoterephthalate, dissolve it in analytically pure methanol, add cuprous chloride and sodium borohydride in sequence, and stir at room temperature for 1-3 hours. The reaction mixture was filtered and concentrated, and column separation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com