Synthesis method and application of novel fluorine-containing oxime ethers
A synthesis method and technology of fluorine-containing oxime ether are applied in the synthesis field of novel fluorine-containing oxime ether, can solve rare problems and the like, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
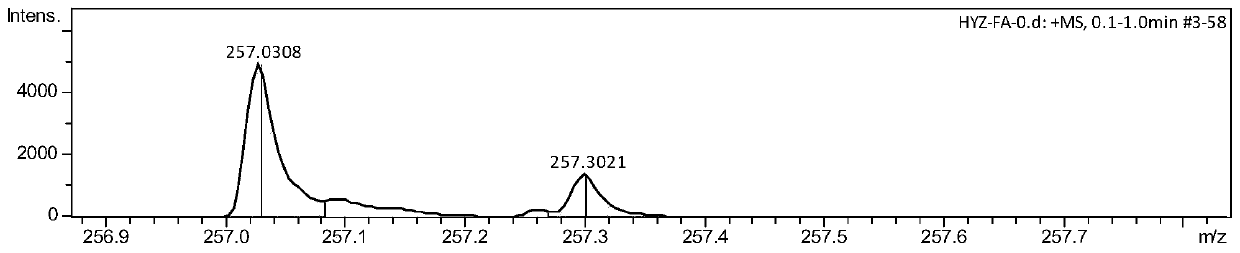
[0052] The synthesis of embodiment 1 fluoroxime ether I
[0053](1) Dissolve 3.0 mmol of raw material oxime 1 (structural formula as follows), 9.0 mmol of potassium carbonate in 15 mL of acetonitrile, stir for 3 hours at room temperature, then add pentafluoropyridine, stir for 12 hours at room temperature, and then spot the plate to confirm the completion of the reaction After that, the reaction solution is obtained;
[0054] (2) The reaction solution was spin-dried in vacuum, extracted 3 times with ethyl acetate and a saturated aqueous sodium chloride solution;
[0055] (3) Eluting with ethyl acetate and petroleum ether with a volume ratio of 2% yielded 596.8 mg of a yellow liquid with a yield of 85%. The product was tested by NMR, IR and mass spectrometry.
[0056] The NMR test results are as follows: 1 H NMR(400MHz,Chloroform-d)δ3.11(t,J=8.0Hz,2H),3.02(t,J=8.0Hz,2H),2.03–2.12(m,2H); 19 F NMR (376MHz, Chloroform-d) δ-90.51–90.34(m),-155.72–155.55(m); 13 C{ 1 H}NMR (100MH...
Embodiment 2
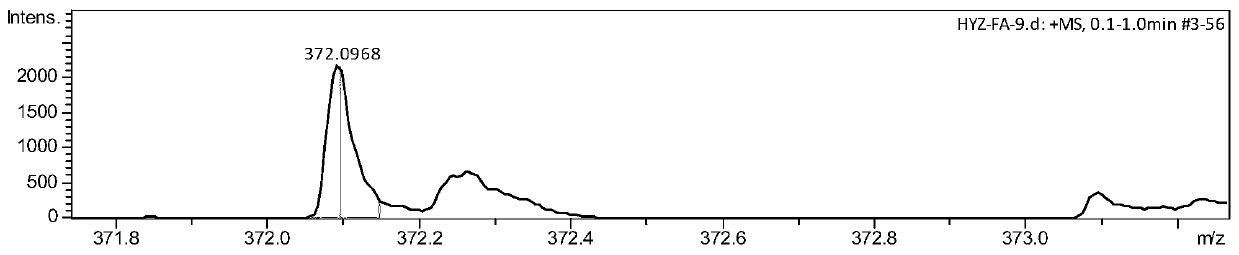
[0061] Embodiment 2 Synthesis of fluoroxime ether II-1
[0062] (1) Dissolve 3.0 mmol of raw material oxime 2 (structural formula as follows), 9.0 mmol of potassium carbonate in 15 mL of acetonitrile, stir and react for 3 hours at room temperature, then add pentafluoropyridine, stir and react for 12 hours at room temperature, and then spot the plate to confirm the completion of the reaction After that, the reaction solution is obtained;
[0063] (2) The reaction solution was spin-dried in vacuum, extracted 3 times with ethyl acetate and a saturated aqueous sodium chloride solution;
[0064] (3) Eluting with ethyl acetate and petroleum ether with a volume ratio of 2% yielded 842.5 mg of a white solid with a yield of 78%. The product was tested by NMR, IR and mass spectrometry.
[0065] NMR test results are: 1 H NMR (400MHz, Chloroform-d) δ7.80–7.86(m,3H),7.69(s,1H),7.45–7.52(m,2H),7.38–7.40(m,1H),3.83–3.91(m ,1H),3.68–3.61(m,1H),3.53–3.61(m,1H),3.27–3.38(m,2H); 19 F NMR (37...
Embodiment 3
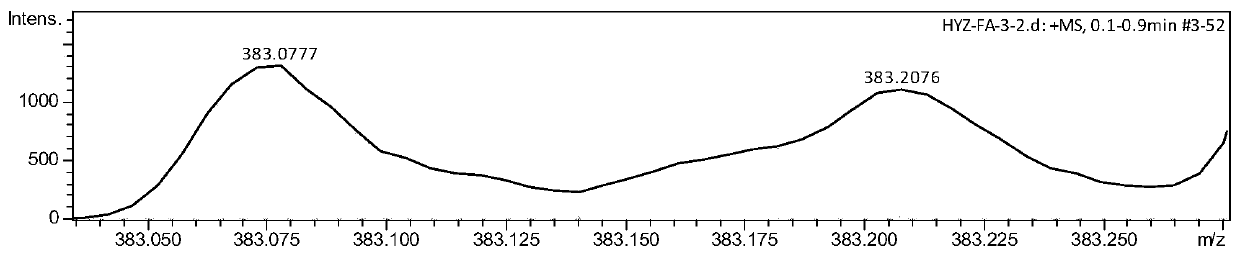
[0070] Embodiment 3 Synthesis of fluoroxime ether II-2
[0071] (1) Dissolve 3.0 mmol of the raw material oxime 3 (structural formula is as follows), 9.0 mmol of potassium carbonate in 15 mL of acetonitrile, stir for 3 hours at room temperature, then add pentafluoropyridine, stir for 12 hours at room temperature, and then spot the plate to confirm the completion of the reaction After that, the reaction solution is obtained;
[0072] (2) The reaction solution was spin-dried in vacuum, extracted 3 times with ethyl acetate and a saturated aqueous sodium chloride solution;
[0073] (3) Eluting with ethyl acetate and petroleum ether with a volume ratio of 2%, 1435.5 mg of a white solid was obtained with a yield of 80%. The product was tested by NMR, IR and mass spectrometry.
[0074] NMR test results are: 1 H NMR (400MHz, Chloroform-d)δ5.02(s,1H),4.31(s,1H),3.56–3.62(m,1H),3.42–3.48(m,1H),3.05–3.15(m,2H ),1.47(s,9H); 19 F NMR (375MHz, Chloroform-d) δ-90.23–90.06(m),-155.57–157....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com