Boron heterocyclic compound, display panel and display device
A display panel and compound technology, applied in the field of OLED, to achieve high thermal stability, improve color purity, and improve luminous stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
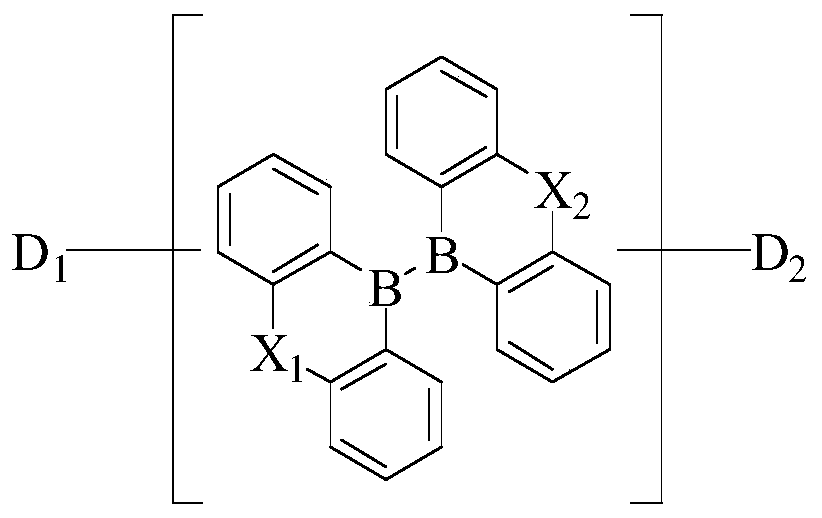
[0026] According to one embodiment of the boron heterocycle compound of the present invention, the boron heterocycle compound has a structure shown in chemical formula II:
[0027]
[0028] According to one embodiment of the boron heterocycle compound of the present invention, the boron heterocycle compound has a structure shown in chemical formula III:
[0029]
[0030] In the boron heterocyclic compound of the present invention, the two boron heterocyclic rings in the chemical structure form a spatially orthogonal structure, so that there are two donor-acceptor light-emitting units in the same molecule, which can significantly increase the oscillator strength of the light-emitting molecule and improve Luminous efficiency. The two boron heterocycles are connected by SP3 hybridization, which reduces the conjugation length of the entire molecule and effectively increases the triplet energy level of the molecule. The spatially orthogonal structure between the two boron he...
Embodiment 1
[0086] Synthesis of Compound H05
[0087]
[0088] S1 (10 mmol) was dissolved in THF (80 mL), cooled to -78°C. Using a dropping funnel, triethylsodium borohydride (10 mL, 1M THF solution) was added dropwise to the above solution for 2 h. After the dropwise addition was completed, stirring was continued for 2h. Then slowly warm to room temperature and stir overnight. The solvent was removed under high vacuum, washed with high-purity THF, and filtered to obtain a colorless solid S2 (3.6 mmol, yield 72%).
[0089] MALDI-TOF MS: C 24 h 14 B 2 f 2 : m / z calculated value: 362.1; tested value: 362.3.
[0090]
[0091] S3 (25 mmol) was dissolved in 50 mL of anhydrous THF at room temperature under nitrogen atmosphere. NaH (30 mmol) was repeatedly washed with n-hexane, and then added to the above solution. After stirring for 1 h, S2 (10 mmol) was added and stirred overnight at room temperature. The reaction was quenched by adding methanol and water. Extract with dichlor...
Embodiment 2
[0095] Synthesis of Compound H29
[0096]
[0097] S4 (12.0 mmol) was dissolved in 50 mL of anhydrous THF at room temperature under nitrogen atmosphere. NaH (15.0 mmol) was repeatedly washed with n-hexane, and then added to the above solution. After stirring for 1 h, S2 (5.0 mmol) was added and stirred overnight at room temperature. The reaction was quenched by adding methanol and water. Extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel chromatography using chloroform / n-hexane as the eluent, and finally purified again by sublimation to obtain solid H29 (3.8 mmol, yield 76%).
[0098] MALDI-TOF MS: C 102 h 70 B 2 N 6: m / z calculated value: 1400.6; tested value: 1400.7.
[0099] Elemental Analysis Calculated: C, 87.42; H, 5.03; B, 1.54; N, 6.00; Tested: C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com