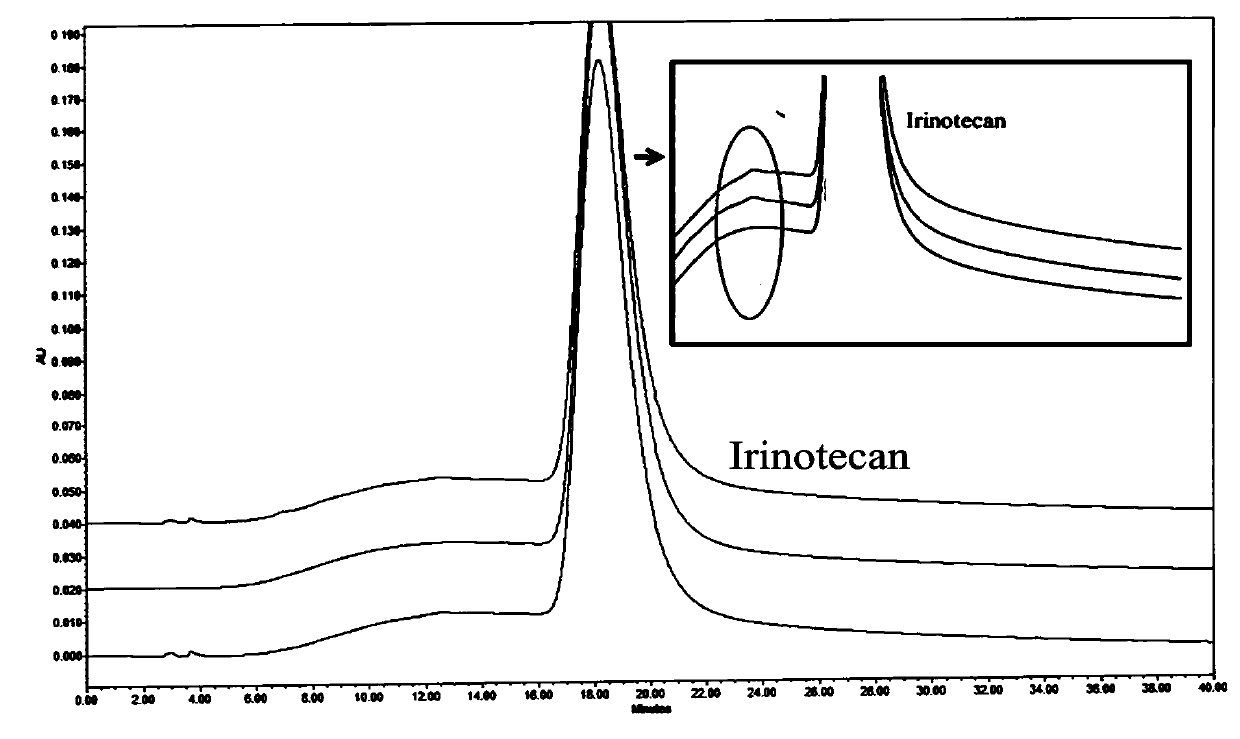
Method for detecting high performance liquid chromatography of isomer in irinotecan hydrochloride injection
A kind of technology of irinotecan hydrochloride, detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Detection conditions:
[0067] Instrument: Waters 2695, Waters 2489 UV detector
[0068] Column: IC (5μm, 4.6×250mm) chiral chromatography column
[0069] Mobile phase: methanol-absolute ethanol-n-hexane-diethylamine (100:100:20:0.2)
[0070] Flow rate: 1.8mL / min
[0071] Column temperature: 35°C
[0072] Injection volume: 25μL
[0073] Detection wavelength: 370nm
[0074] Experimental steps:
[0075] Diluent preparation: 0.05% phosphoric acid in absolute ethanol
[0076] Take about 20mg of irinotecan hydrochloride trihydrate reference substance in a 100mL measuring bottle, take the diluent prepared above, add to about 2 / 3 volume of the measuring bottle, dissolve it by ultrasonic, dilute to the mark with the diluent, shake well, and use as stock solution-1.
[0077] Take about 5.4mg of irinotecan hydrochloride trihydrate isomer reference substance in a 25mL measuring bottle, add diluent to about 2 / 3 volume of the measuring bottle, dissolve it by ultrasonic, di...
Embodiment 2
[0081] Take about 20mg of irinotecan hydrochloride trihydrate reference substance in a 100mL measuring bottle, use 0.05% phosphoric acid in absolute ethanol as the diluent, add the diluent to about 2 / 3 volume of the measuring bottle, ultrasonically dissolve it, and use the diluent Dilute to the mark, shake well, and use as the stock solution of the test substance. Take 4.0mL of the stock solution of the test product to a 250mL measuring bottle, dilute to the mark with diluent, shake well, and use it as the test solution.
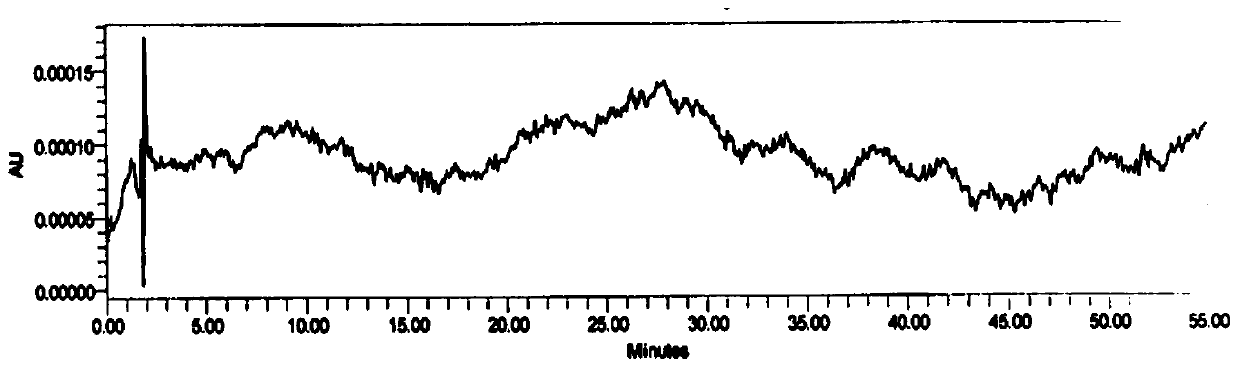
[0082] Accurately measure 25 μ L of need testing solution, detect and analyze by the detection condition of embodiment 1, see Figure 4 .
[0083] Figure 4 It showed that no isomer was detected in the test product.
Embodiment 3
[0085] Get irinotecan hydrochloride injection (prepared according to CN109908077A embodiment 1) 2mL in 100mL measuring bottle, take the dehydrated ethanol solution of 0.05% phosphoric acid as diluent, dilute to scale, shake up, be mixed with need testing solution.
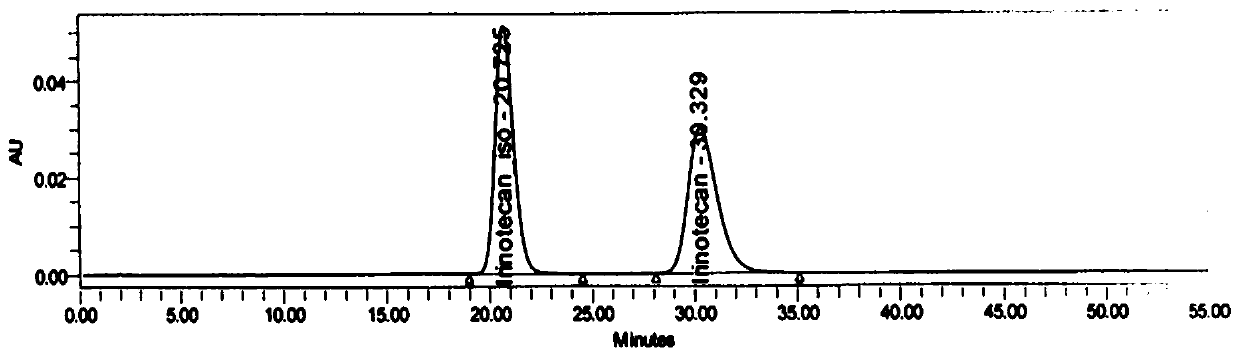
[0086] Precisely measure 25 μ L of the test solution prepared in Example 3, and detect and analyze according to the detection conditions of Example 1, see Figure 5 .
[0087] Figure 5 It shows that the analysis method of the present invention can effectively and accurately separate irinotecan hydrochloride and its isomers in irinotecan hydrochloride injection. After the method is optimized, the unknown impurity peak no longer interferes with the determination of isomer impurities. In the stability test , the isomer content did not increase significantly. The optical purity of this product has reached the quality requirements, indicating that this method is suitable for the detection of isomer impurities in irin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com