Gastric retention long acting preparation and preparation method thereof
A gastric retention and long-acting technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, etc. It can solve problems such as short retention time, complicated production process, and uneven drug release. , to achieve the effect of easy control of the production process, stable and reliable quality, and suitable for large-scale batch production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 4mg of huperzine A and 1.5g of hydroxypropylmethylcellulose K100M, take 0.5ml of 75% ethanol to dissolve huperzine A, pour it into K100M evenly, and mix well to obtain a soft material; use a special mold to press Become the sustained-release drug microtablet of diameter 1.9mm, dry for subsequent use.
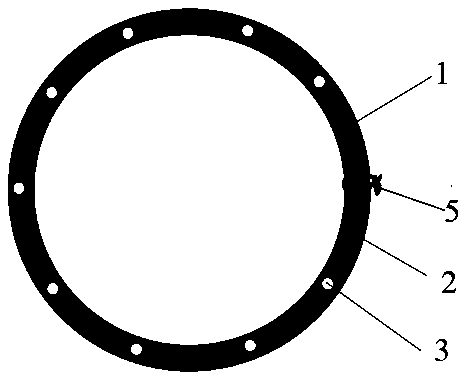
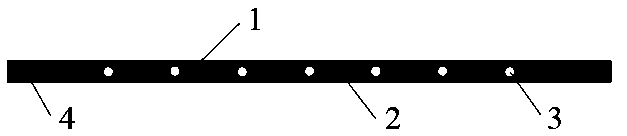
[0036] Take a medical sterile silicone tube with a length of 9cm (the inner diameter of the longitudinal section is 2mm, the outer diameter is 3mm), and one side of the tube wall is opened with a hole diameter of 0.5mm, and holes are evenly opened between the 0.5cm to 8.5cm section of the silicone tube 7 pieces; the 0.5cm to 8.5cm section of the open-hole silicone tube is filled with sustained-release drug microchips, and the space 0.5cm away from both ends is sealed by filling the silicone rubber special adhesive; the silicone section is sutured and sealed with degradable sutures, A closed drug-containing silicone ring is formed, and the silicone ring is folded and...
Embodiment 2
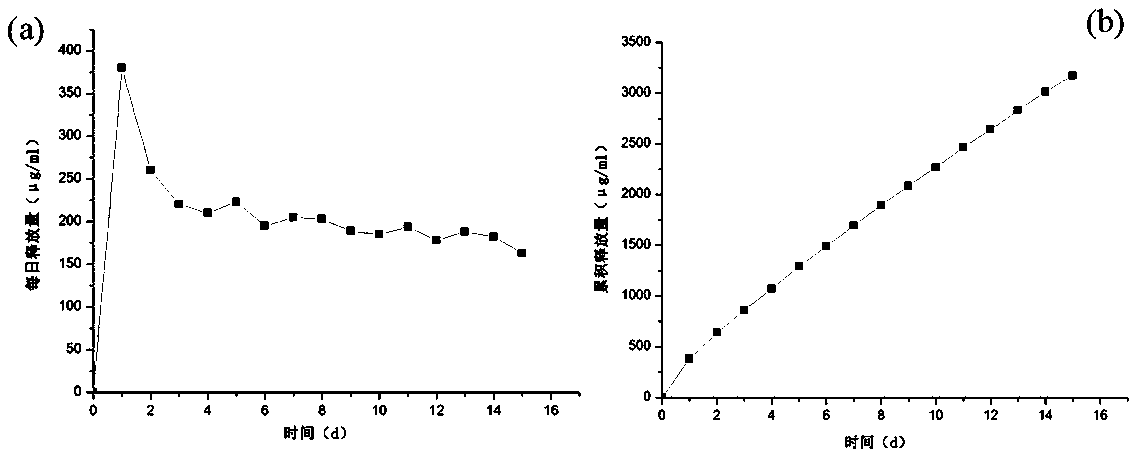
[0038]According to the method of Example 1, a long-acting gastric retention preparation containing 4 mg of huperzine A was prepared, and the resulting gastric retention long-acting preparation was put into a glass bottle, 10 ml of water was added, the bottle stopper was sealed, and 6 groups were parallelized. Put it into a shaking table, the rotating speed of the shaking table is 75r / min, measure the absorbance value by ultraviolet light every day, then change the water 10ml, continue to put it into the shaking table, and measure for 15 days. Record daily measurement data, calculate the average value, draw daily release and cumulative release curves, the results are as follows image 3 shown.
Embodiment 3
[0040] Weigh 4mg of huperzine A and 1.5g of hydroxypropyl methylcellulose K100M, take 0.5ml of 75% ethanol to dissolve huperzine A, pour it into K100M evenly, and mix well to obtain a soft material; use a special mold Press into sustained-release drug microtablets with a diameter of 1.9 mm, and dry for subsequent use.
[0041] Take a medical sterile silicone tube with a length of 9cm (the inner diameter of the longitudinal section is 2mm, the outer diameter is 3mm), and one side of the tube wall is opened with a hole diameter of 0.5mm, and holes are evenly opened between the 0.5cm to 8.5cm section of the silicone tube 9 pieces; the 0.5cm to 8.5cm section of the open-hole silicone tube is filled with sustained-release drug microchips, and the space 0.5cm away from both ends is sealed by filling the silicone rubber special adhesive; the silicone section is sutured and sealed with degradable sutures, A closed drug-containing silicone ring is formed, and the silicone ring is folde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com