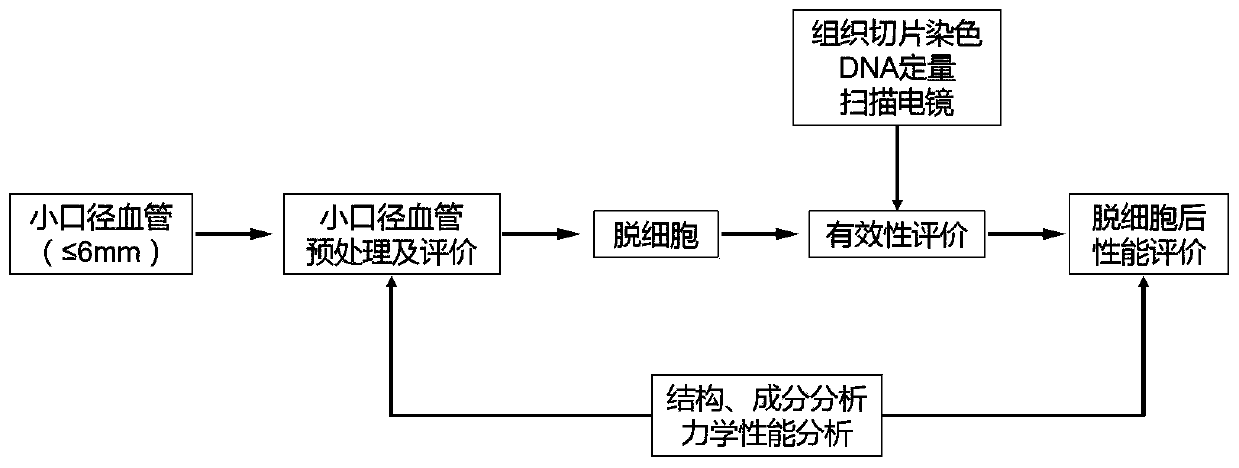
Small-caliber vascular stent for decellularization and preparation method of small-caliber vascular stent
A vascular stent and small-caliber technology, which is applied in the field of preparation of decellularized small-caliber vascular stents, can solve the problems of inflammatory immune rejection, changes in the mechanical properties of the decellularized solution, etc., to reduce immunogenicity, reduce nuclear substances and antigenic components, Conducive to the effect of adhesion growth, proliferation and migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation method of decellularized human umbilical artery stent
[0037] Experimental material: human umbilical artery
[0038] Equipment: ultra-clean table, shaker, sterile biochemical incubator
[0039] The specific cultivation method includes the following steps:
[0040](1) Obtaining of umbilical artery: The umbilical cords of newborns with good health and gestational age (38-42 weeks) were obtained, and the umbilical cords were obtained with the informed consent of the puerpera and voluntary donation (the research protocol was approved by the Ethics Committee of Guangdong Provincial People’s Hospital). 4-6cm, diameter 2-3mm, the following operations are all performed under the sterile environment of ultra-clean bench and biochemical incubator;
[0041] (2) Pretreatment of the umbilical artery: carefully peel off the connective tissue around the umbilical artery to avoid damage to the adventitia of the umbilical artery, rinse the separated umbilical ar...
Embodiment 2
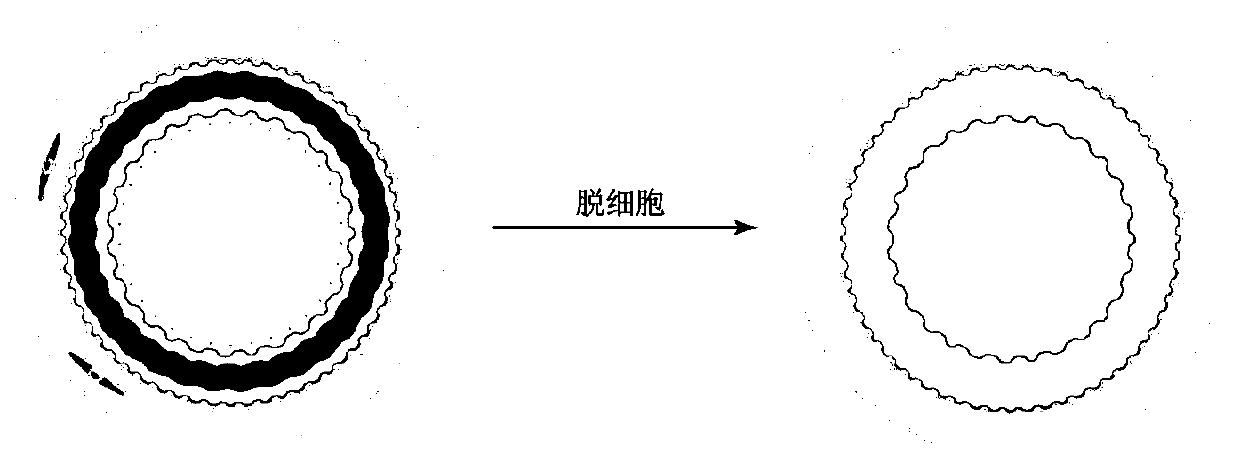
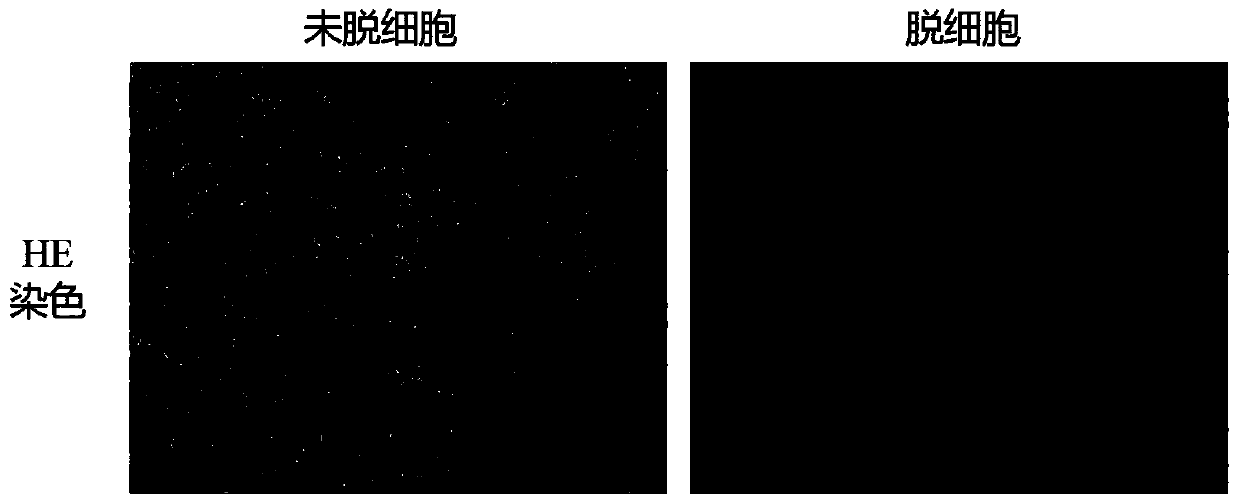
[0045] Example 2 Evaluation of the Effectiveness of the Decellularized Human Umbilical Artery Stent
[0046] HE staining of human umbilical arteries before and after decellularization
[0047] The decellularized umbilical artery stent prepared in the embodiment of the present invention and the umbilical artery of the control group not treated with chemical detergent were subjected to HE staining, and the specific steps were as follows: two groups of umbilical arteries were immersed in 4% paraformaldehyde and fixed overnight, The tissue was taken out the next day, washed twice with PBS, 15min / time, dehydrated with 50%, 70%, 80%, 90%, 95%, 100%, 100% ethanol gradient, then immersed in xylene for 20min; The umbilical arteries of the group were fully immersed in hot-melted paraffin (54°C-56°C), embedded in paraffin in the mold, and after the paraffin solidified after cooling, the embedding block was taken out, paraffin sectioned, cut into 4mm, and exhibited in a water bath at 40°C...
Embodiment 3
[0050] Example 3 Component and Structure Detection of Decellularized Human Umbilical Artery Stent
[0051] Scanning electron microscopy of human umbilical arteries before and after decellularization
[0052] The decellularized umbilical artery stent prepared in the embodiment of the present invention and the umbilical artery of the control group not treated with chemical detergent were subjected to scanning electron microscope experiments. The specific steps were as follows: two groups of umbilical arteries were placed in 2.5% glutaraldehyde solution Fix at 4°C for 4h, discard the fixative, rinse the sample with PBS, then dehydrate with ethanol gradient, replace the ethanol twice with isoamyl acetate, freeze the specimen at -20°C, -40°C, and -80°C for 12h, and place them in a freeze dryer. Dry in medium for 12h, and prepare the specimens for later use. Metal spraying was carried out before observation with a scanning electron microscope. In order to make the sample conductive...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com