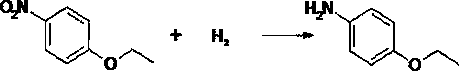Green synthesis method of p-aminophenetole
A technology for the green synthesis of p-aminophenethyl ether, which is applied in the preparation of aminohydroxyl compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of environmental hazards, affecting product purity, and high yield, so as to improve product purity. , the environment and operators are safe and reliable, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] First use nitrogen (<1MPa) to continuously purge the 5000L reduction reactor for 3 times, and discharge the purge gas through the vent pipe. Under the condition of no solvent, add 2000kg of p-nitrophenethyl ether into the reaction kettle, add 2kg of Raney nickel catalyst, use steam to raise the temperature to 80°C, feed hydrogen, and keep the heat and pressure (80~90°C, 1.5~2.5 MPa ) under the condition of hydrogenation reduction reaction for 6 hours. After the reaction, purging with nitrogen (<1MPa) continuously for 3 times, at 65°C, filter under normal pressure to recover the catalyst, and rectify the filtrate for 120 hours under negative pressure to obtain 1600kg of p-aminophenethyl ether, 20kg of o-aminophenethyl ether, Aminophenethyl ether 60kg, the distillation residue is handled by a qualified unit.
Embodiment 2
[0022] First use nitrogen (<1MPa) to continuously purge the 5000L reduction reactor for 3 times, and discharge the purge gas through the vent pipe. Under solvent-free conditions, add 2001kg of p-nitrophenethyl ether into the reaction kettle, add 4kg of Raney nickel catalyst, use steam to raise the temperature to 80°C, pass in hydrogen, and keep the temperature and pressure (80-90°C, 1.5-2.5 MPa ) under the condition of hydrogenation reduction reaction for 6 hours. After the reaction, purging with nitrogen (<1MPa) continuously for 3 times, at 65°C, filter under normal pressure to recover the catalyst, and rectify the filtrate for 120 hours under negative pressure to obtain 1610kg of p-aminophenethyl ether, 20kg of o-aminophenethyl ether, Aminophenetole 50kg, the distillation residue is handled by a qualified unit.
Embodiment 3
[0024] First use nitrogen (<1MPa) to continuously purge the 5000L reduction reactor for 3 times, and discharge the purge gas through the vent pipe. Add 2002kg of p-nitrophenethyl ether into the reaction kettle, add 2.5kg of Raney nickel catalyst, use steam to raise the temperature to 80°C, feed hydrogen, and carry out heating under the conditions of heat preservation and pressure (80-90°C, 1.5-2.5 MPa). The hydrogen reduction reaction was carried out for 6 hours. After the reaction, purging with nitrogen (<1MPa) continuously for 3 times, at 65°C, filter under normal pressure to recover the catalyst, and rectify the filtrate for 120 hours under negative pressure to obtain 1620kg of p-aminophenethyl ether, 20kg of o-aminophenethyl ether, Aminophenetole 40kg, the distillation residue is handled by a qualified unit.
[0025] The whole process of the present invention adopts solvent-free process conditions, which reduces the discharge of organic matter in the traditional productio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com