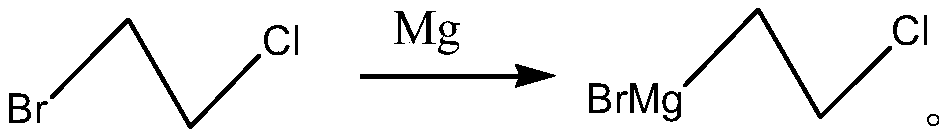Preparation method of (S)-N-BOC-3-hydroxypiperidine
A technology of -N-BOC-3-, hydroxypiperidine, which is applied in the field of preparation of -N-BOC-3-hydroxypiperidine, can solve the problems of cumbersome post-processing, high price, and high price of biological enzymes, and achieve the preparation method Environmental protection, good product quality, high reaction safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] In this embodiment, a preparation method of (S)-N-BOC-3-hydroxypiperidine is provided, and the preparation method comprises the following steps:
[0062] (1) Preparation of 2-chloroethylmagnesium bromide Grignard reagent. A mixture of 1-bromo-2-ethane (177.5 g, 1.25 mol) and 500 mL of tetrahydrofuran was used for later use.
[0063] Under nitrogen protection, 29.16 g (1.2 mol) of magnesium bars, 300 mL of tetrahydrofuran, and a grain of iodine were sequentially added, and 20 mL of the 1-bromo-2-chloroethane tetrahydrofuran solution prepared above was added dropwise. Heat slowly to reflux. When the reaction night faded, the remaining 1-bromo-2-chloroethane tetrahydrofuran solution was added dropwise. After dripping, the reaction was continued for 2 hours under reflux. After cooling to room temperature, the obtained 2-chloroethylmagnesium bromide Grignard reagent was protected by nitrogen for use.
[0064] (S)-epichlorohydrin (92.5g, 1mol) and 500mL of anhydrous tetra...
Embodiment 2
[0070] (1) Prepare 2-chloroethylmagnesium bromide Grignard reagent according to Example 1 for subsequent use.
[0071] (S)-epichlorohydrin (92.5g, 1mol) and 500mL of anhydrous tetrahydrofuran were stirred and mixed, cooled to -65°C, and the 2-chloroethylmagnesium bromide Grignard reagent prepared above (167.5g, 1mol) was added dropwise. . The reaction is exothermic. The dropping rate was controlled so that the reaction temperature was not higher than -55°C. After dripping, the reaction was incubated for 1.5 hours. Turn off the freezer and let it naturally warm to room temperature. Then 400 grams of saturated aqueous ammonium chloride solution was added dropwise. After stirring at room temperature for 1 hour, the reaction solution was extracted three times with 500 mL of ethyl acetate each time. The organic phases were combined, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to remove the solvent, and distilled in high vacuum to obtain 120.18 ...
Embodiment 3
[0076] (1) Prepare 2-chloroethylmagnesium bromide Grignard reagent according to Example 1 for subsequent use.
[0077] (S)-epichlorohydrin (92.5 g, 1 mol) and 500 mL of anhydrous tetrahydrofuran were stirred and mixed, cooled to -30°C, and the 2-chloroethylmagnesium bromide Grignard reagent prepared above (335 g, 2 mol) was added dropwise. The reaction is exothermic. The dropping rate was controlled so that the reaction temperature was not higher than -20°C. After dripping, the reaction was incubated for 2.5 hours. Turn off the freezer and let it naturally warm to room temperature. Then 400 grams of saturated aqueous ammonium chloride solution was added dropwise. The mixture was stirred at room temperature for 2 hours, and the reaction solution was 500 mL×3 of ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to remove the solvent, and distilled in high vacuum to obtain 99.23 g of (S)-1,5-dichloro-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com