Preparation method for Mannich base containing ferrocenyl thiadiazole or oxadiazole
A technology based on thiadiazolyl and oxadiazolyl is applied in the field of preparation of ferrocene-based thiadiazole or oxadiazolyl-based Mannich base, and can solve the problem that catalysts pollute the environment, are difficult to recycle, and have low catalytic efficiency. and other problems, to achieve the effect of simple post-processing, simple operation and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
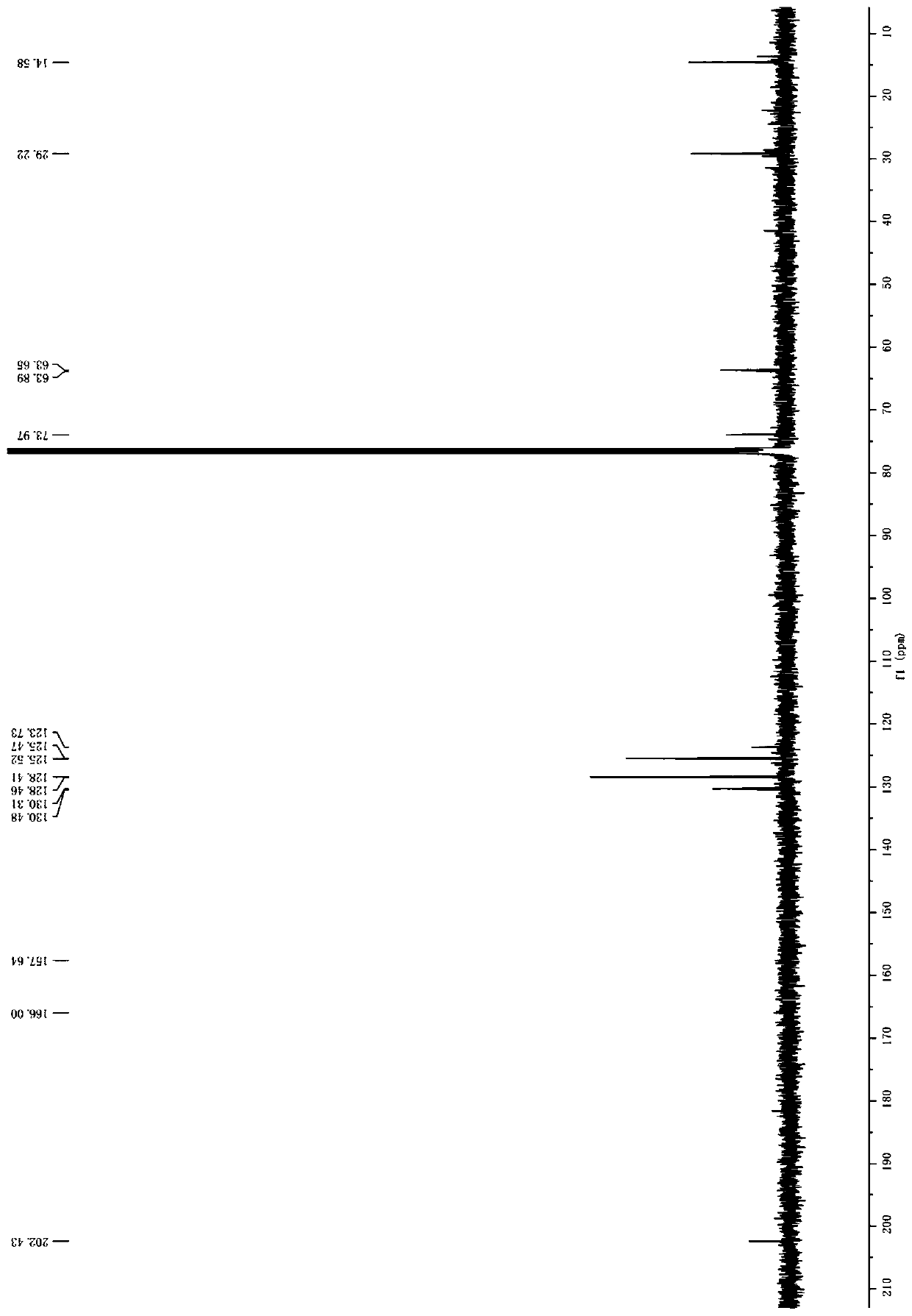
[0034] Example 1 Preparation of 1-(5'-phenyl-1',3',4'-thiadiazole-2'-amino)-ethyl-ferrocenyl ketone
[0035] Add 2-amino 5-phenyl-1,3,4-thiadiazole (1mol), 37% formaldehyde (5mol) solution to a dry three-necked flask, and absolute ethanol as a solvent, stir well; add benzothiazolium ion liquid (1.2mol), then slowly add acetylferrocene (1mol) absolute ethanol solution dropwise, and heat to reflux until the reaction ends (monitored by TLC), evaporate the solvent under reduced pressure, add ethyl acetate to the residue, Suction filtration, the filter cake is benzothiazole ionic liquid, which can be recycled and reused; the filtrate filtrate is evaporated to dryness to obtain the crude product, which is recrystallized from absolute ethanol to obtain 1-(5'-phenyl-1',3',4' -Thiadiazole-2'-amino)-ethyl-ferrocenyl ketone, m.p.196-198°C, yield: 91.8%.
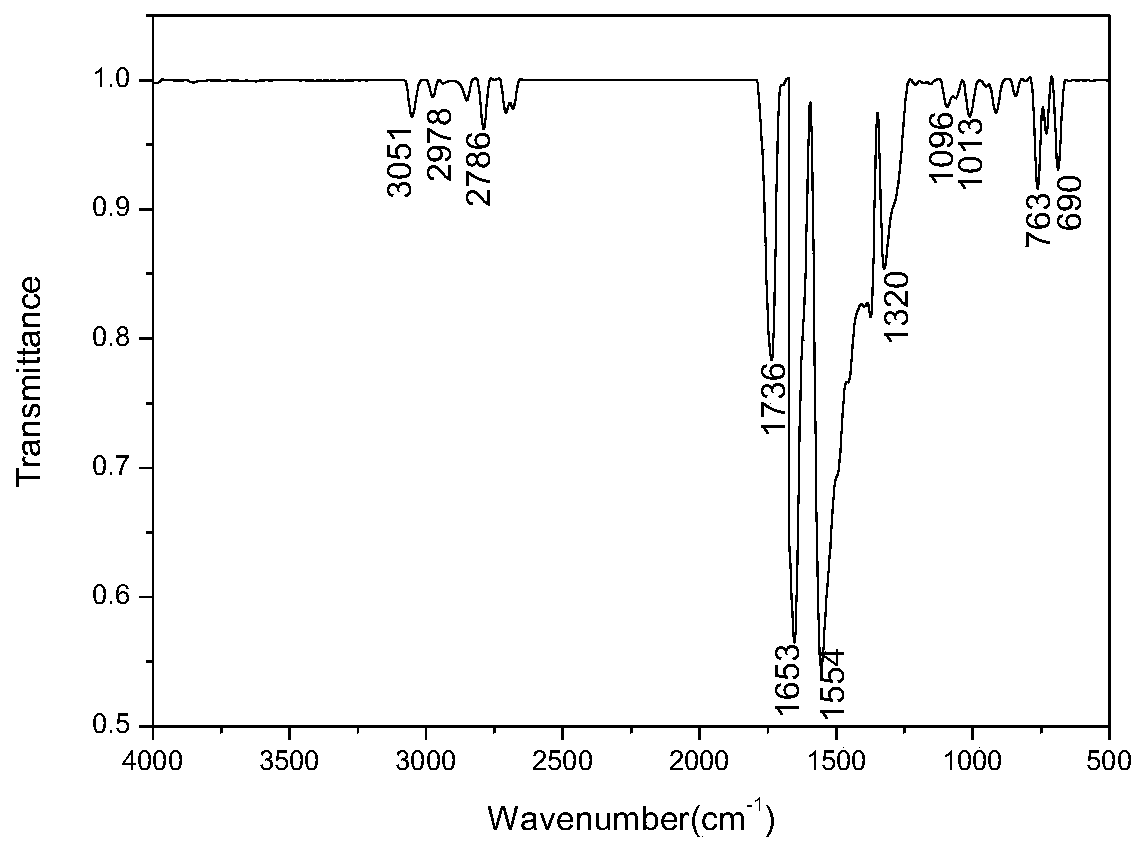
[0036] IR(KBr)v:3324(ν Ph-H ),2914(ν 饱和C -H),1730(ν C=O ),1611(ν C=C ),1365(ν C-N ),806(γ 1,4-Ph-H ).
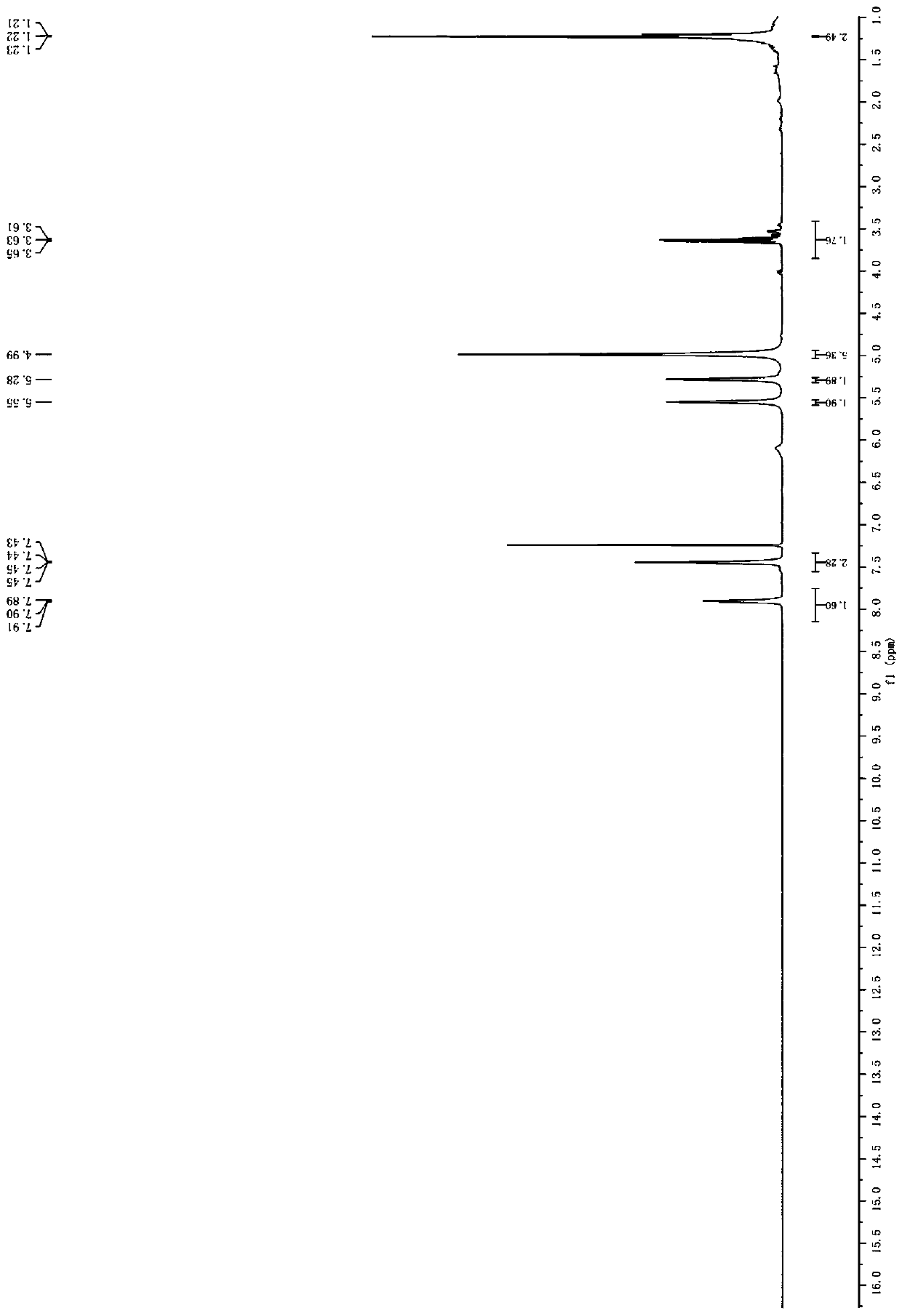
[0037] 1 HNMR(DMSO,40...
Embodiment 2
[0039] Example 2 Preparation of 1-(5'-p-chlorophenyl-1',3',4'-thiadiazole-2'-amino)-ethyl-ferrocenyl ketone
[0040]Add 2-amino 5-p-chlorophenyl-1,3,4-thiadiazole (1mol) and 37% formaldehyde (5mol) solution to a dry three-necked flask, and use absolute ethanol as a solvent, stir well; add benzo Thiazole ionic liquid (1.2mol), then slowly add acetyl ferrocene (1mol) absolute ethanol solution dropwise, heat and reflux until the end of the reaction (monitored by TLC), evaporate the solvent under reduced pressure, and add ethyl acetate to the residue Ester, suction filtration, the filter cake is benzothiazole ionic liquid, which can be recycled and reused; the filtrate is evaporated to dryness to obtain the crude product, which is recrystallized from absolute ethanol to obtain 1-(5'-p-chlorophenyl-1',3' ,4'-thiadiazole-2'-amino)-ethyl-ferrocenyl ketone, m.p.170-172°C, yield: 90.8%.
[0041] IR(KBr)v:3310(ν Ph-H ),2999(ν 饱和C-H ),1708(ν C=O ),1600(ν C=C ),1320(ν C-N ),774(γ 1...
Embodiment 3
[0044] Example 3 Preparation of 1-(5'-p-nitrophenyl-1',3',4'-thiadiazole-2'-amino)-ethyl-ferrocenyl ketone
[0045] Add 2-amino 5-p-nitrophenyl-1,3,4-thiadiazole (1mol) and 37% formaldehyde (5mol) solution in a dry three-necked flask, and dehydrated alcohol is used as a solvent, stir well; add benzene and thiazole ionic liquid (1.2mol), then slowly add acetyl ferrocene (1mol) absolute ethanol solution dropwise, and heat to reflux until the end of the reaction (monitored by TLC), evaporate the solvent under reduced pressure, and add acetic acid to the residue Ethyl ester, suction filtration, the filter cake is benzothiazole ionic liquid, which can be recycled and reused; the filtrate is evaporated to dryness to obtain the crude product, which is recrystallized from absolute ethanol to obtain 1-(5'-p-nitrophenyl-1', 3',4'-Thiadiazole-2'-amino)-ethyl-ferrocenyl ketone. m.p. 199-201°C, yield: 92.3%.
[0046] IR(KBr)v:3288(ν Ph-H ),2994(ν 饱和C-H ), 1759 (ν C=O ),1600(ν C=C ),1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com