Isoguanosine intermediate, preparation method thereof, isoguanosine compound, preparation method thereof and downstream product thereof
A technology of purine nucleoside intermediates and purine nucleoside compounds, which is applied in the field of drug synthesis, can solve the problems of low conversion rate, difficult purification of intermediates, and poor resistance to enzymatic degradation, so as to reduce the generation of isomers and improve Effect of cell uptake rate and ease of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The embodiment of the present invention also provides a method for preparing the above-mentioned isoguanosine intermediate, comprising the following steps:
[0065] Substitution reaction of 2,6-di-amino-purine nucleoside and halogenated alkanes to form the compound represented by formula (1).
[0066] Specifically, 2,6-di-amino-purine nucleoside is reacted with hydroxide to form an active intermediate; specifically, 2,6-di-amino-purine nucleoside is mixed with an organic solvent, and then the mixed solution is The temperature is controlled between 40-100 ° C, and then reacted with hydroxide for 1-3 hours under a protective gas atmosphere to form an active intermediate;
[0067] Preferably, the temperature is controlled at 50-80°C, the most optimal is 60°C;
[0068] Preferably, 5-10 liters of organic solvent is added corresponding to every kilogram of 2,6-di-amino-purine nucleoside;
[0069] Preferably, the organic solvent comprises a polar solvent;
[0070] More pref...
Embodiment 1
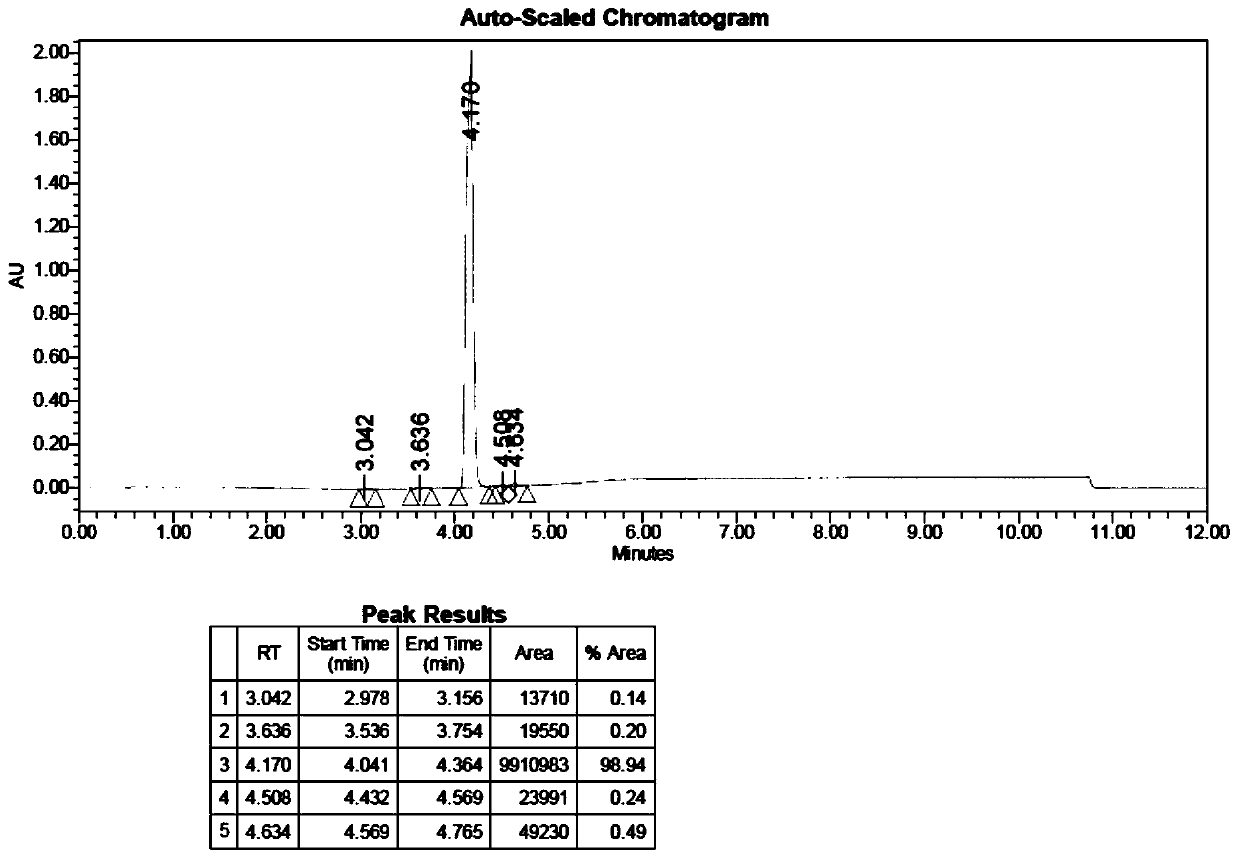
[0108] This embodiment provides a kind of isoguanosine intermediate, its structural formula is as shown in formula (3);
[0109] Formula (3), its high liquid analysis diagram see figure 1 .
[0110] This embodiment also provides a preparation method of the isoguanosine intermediate shown in the above formula (3), comprising the following steps:
[0111] Synthesize isoguanosine intermediate according to the following formula:
[0112]
[0113] The specific operation is as follows;
[0114] Weigh 13kg of 2,6-diaminopurine nucleoside (CAS No.: 2096-10-8) and dissolve it in 1300L DMF, heat the reaction solution to 60°C, add 0.52kg of KOH under the protection of argon, and stir at 60°C for 2 Hour. Then, 19.2 kg of 1-bromotetradecane was added dropwise to the system, and after the drop was completed, the reaction was stirred at 60°C. React until the product no longer increases. Concentrate in vacuo at 65 DEG C until not dripping, and the residual solid is crystallized in ...
Embodiment 2
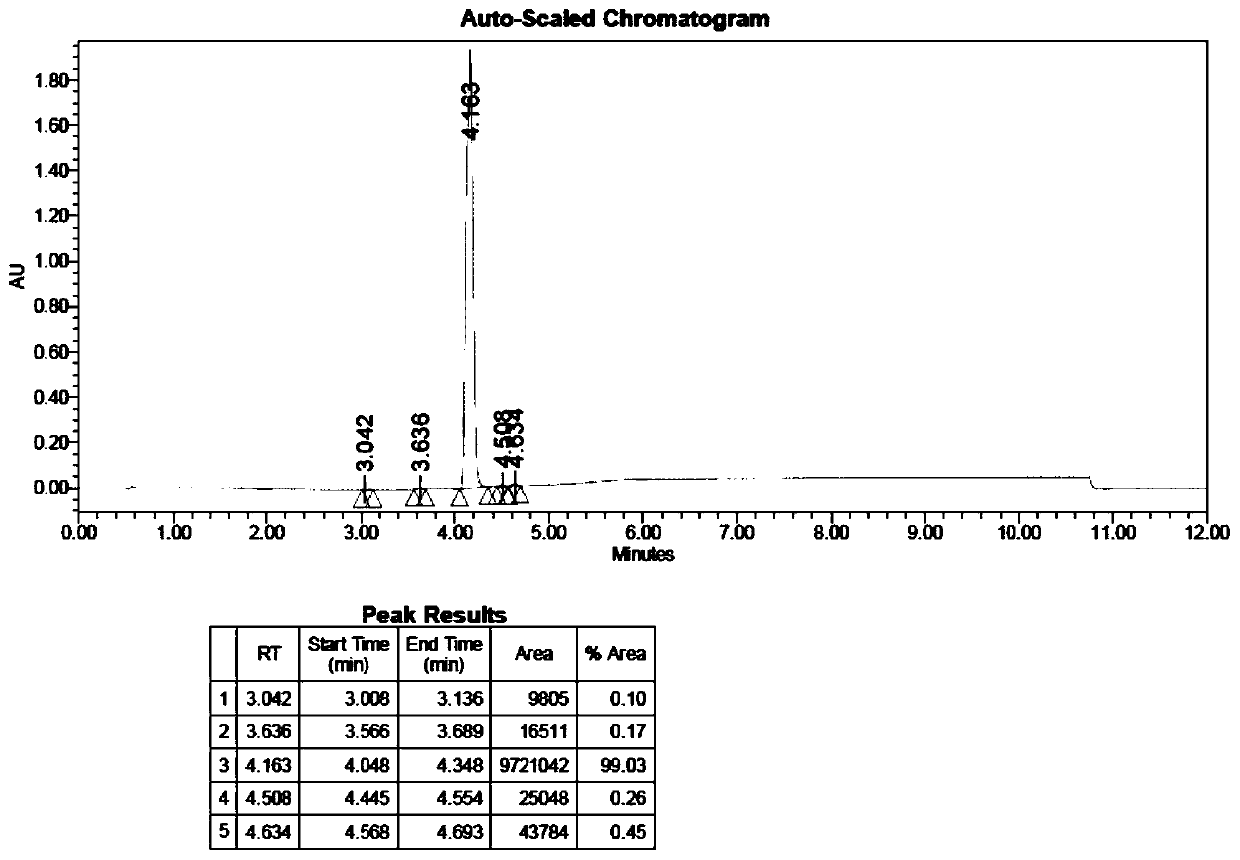
[0132] This embodiment provides an isoguanosine intermediate, the structural formula of which is shown in formula (5);
[0133]
[0134] This embodiment also provides a preparation method of the isoguanosine intermediate shown in the above formula (5), comprising the following steps:
[0135] Synthesize isoguanosine intermediate according to the following formula:
[0136]
[0137] The specific operation is as follows;
[0138] Weigh 13kg of 2,6-diaminopurine nucleoside (CAS No.: 2096-10-8) and dissolve it in 1300L DMF, heat the reaction solution to 60°C, add 0.52kg of KOH under the protection of argon, and stir at 60°C for 2 Hour. Then, 17.2 kg of 1-bromododecane was added dropwise to the system, and the reaction was stirred at 60° C. after the drop was completed. React until the product no longer increases. Then concentrated in vacuo at 65°C until no dripping, the residual solid was crystallized in ethanol (80% by volume of ethanol, and 260kg of ethanol was used) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com