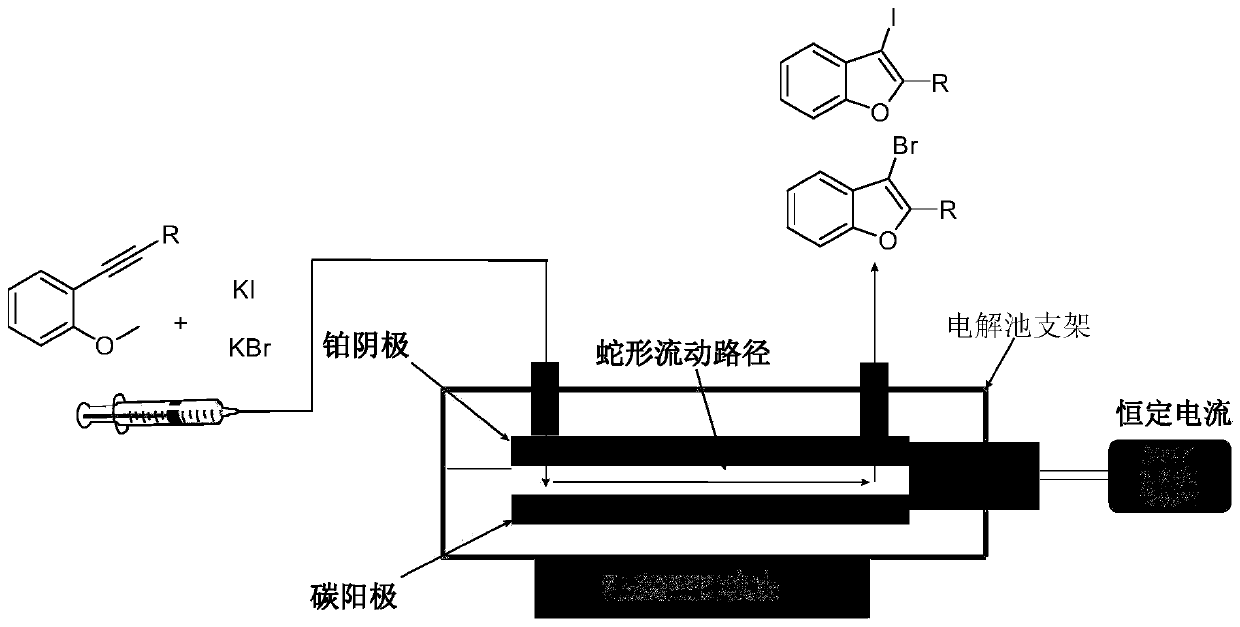
Method for continuously preparing 2-aryl-3-halogenated-benzofuran compounds by using electrochemical microchannel reaction device
A technology of microchannel reaction and benzofuran, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of high reactor design requirements, poor selectivity, environmental pollution, etc., and achieve good industrial application prospects, The effect of low reaction temperature and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
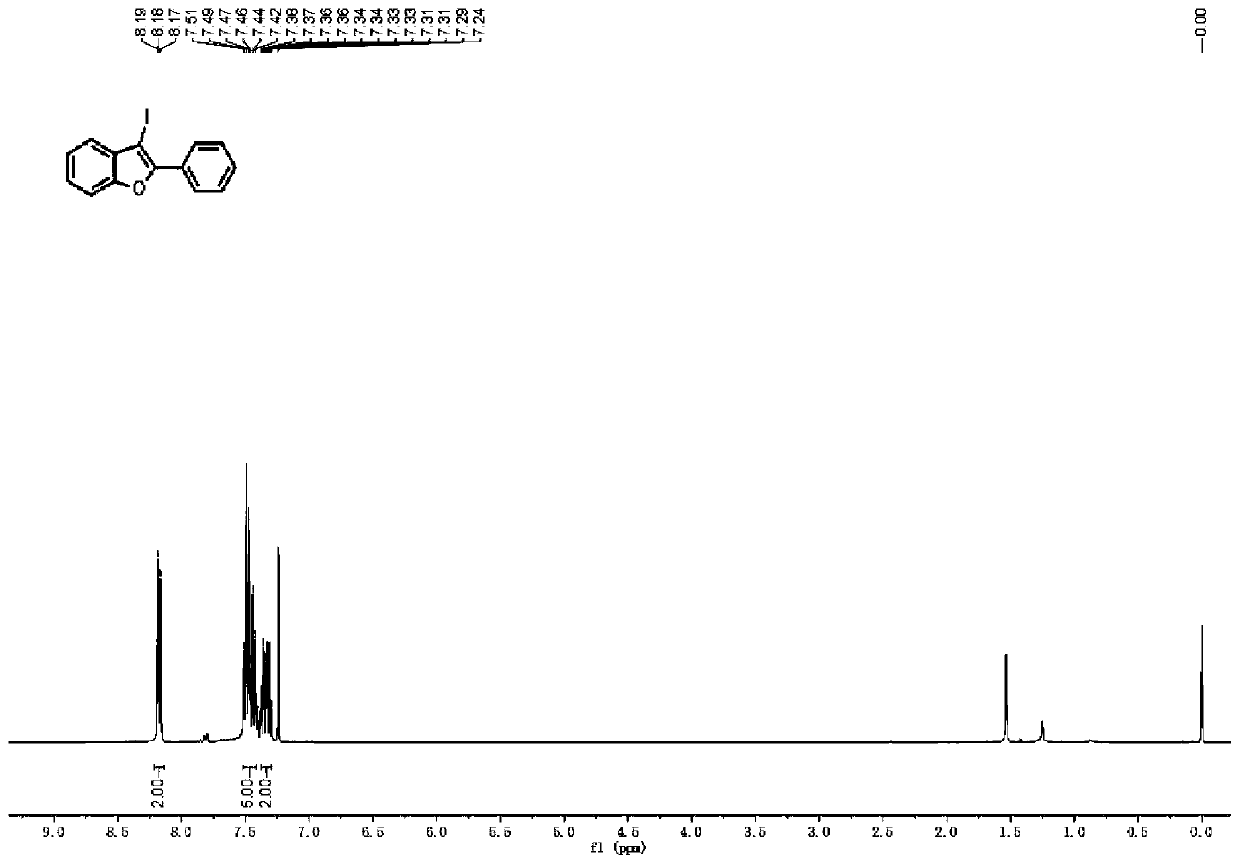
[0032] Assemble the electrochemical flow cell device: select the carbon sheet as the anode electrode, place it on the lower layer of the titanium alloy electrolytic cell bracket, and then place the PTFE reaction tank with a volume of 0.5ml on the upper layer of the carbon sheet, and then plate the cathode with platinum The titanium alloy plate is placed on the upper layer of the reaction tank, and finally fixed with a polytetrafluoroethylene screw and connected to an adjustable DC power supply. Weigh 0.0416g of 2-phenylethynylanisole and 0.0996g of KI and dissolve in 1ml of water and 4ml of acetonitrile to prepare a homogeneous solution A. The prepared homogeneous solution A was passed into the reaction module with a single-stream injection at a flow rate of 0.1 ml / min using a syringe pump. Turn on the power supply, adjust the current to 40 mA, and collect the product 2-phenyl-3-iodo-benzofuran from the outlet of the reaction module after the current is stable. The H NMR spec...
Embodiment 2
[0035] Assemble the electrochemical flow cell device: select the carbon sheet as the anode electrode, place it on the lower layer of the titanium alloy electrolytic cell bracket, and then place the PTFE reaction tank with a volume of 0.5ml on the upper layer of the carbon sheet, and then plate the cathode with platinum The titanium alloy plate is placed on the upper layer of the reaction tank, and finally fixed with a polytetrafluoroethylene screw and connected to an adjustable DC power supply. Weigh 0.0416g of 2-phenylethynyl anisole and 0.09g of NaI and dissolve in 1ml of water and 4ml of acetonitrile to prepare a homogeneous solution A. The prepared homogeneous solution A was passed into the reaction module with a single-stream injection at a flow rate of 0.1 ml / min using a syringe pump. Turn on the power supply, adjust the current to 40 mA, and collect the product 2-phenyl-3-iodo-benzofuran from the outlet of the reaction module after the current is stable.
Embodiment 3
[0037] Assemble the electrochemical flow cell device: select the carbon sheet as the anode electrode, place it on the lower layer of the titanium alloy electrolytic cell bracket, and then place the PTFE reaction tank with a volume of 0.5ml on the upper layer of the carbon sheet, and then plate the cathode with platinum The titanium alloy plate is placed on the upper layer of the reaction tank, and finally fixed with a polytetrafluoroethylene screw and connected to an adjustable DC power supply. Weigh 0.0416g of 2-phenylethynyl anisole and 0.222g of Bu 4 NI was dissolved in 1 ml of water and 4 ml of acetonitrile to make a homogeneous solution A. The prepared homogeneous solution A was passed into the reaction module with a single-stream injection at a flow rate of 0.1 ml / min using a syringe pump. Turn on the power supply, adjust the current to 40 mA, and collect the product 2-phenyl-3-iodo-benzofuran from the outlet of the reaction module after the current is stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com