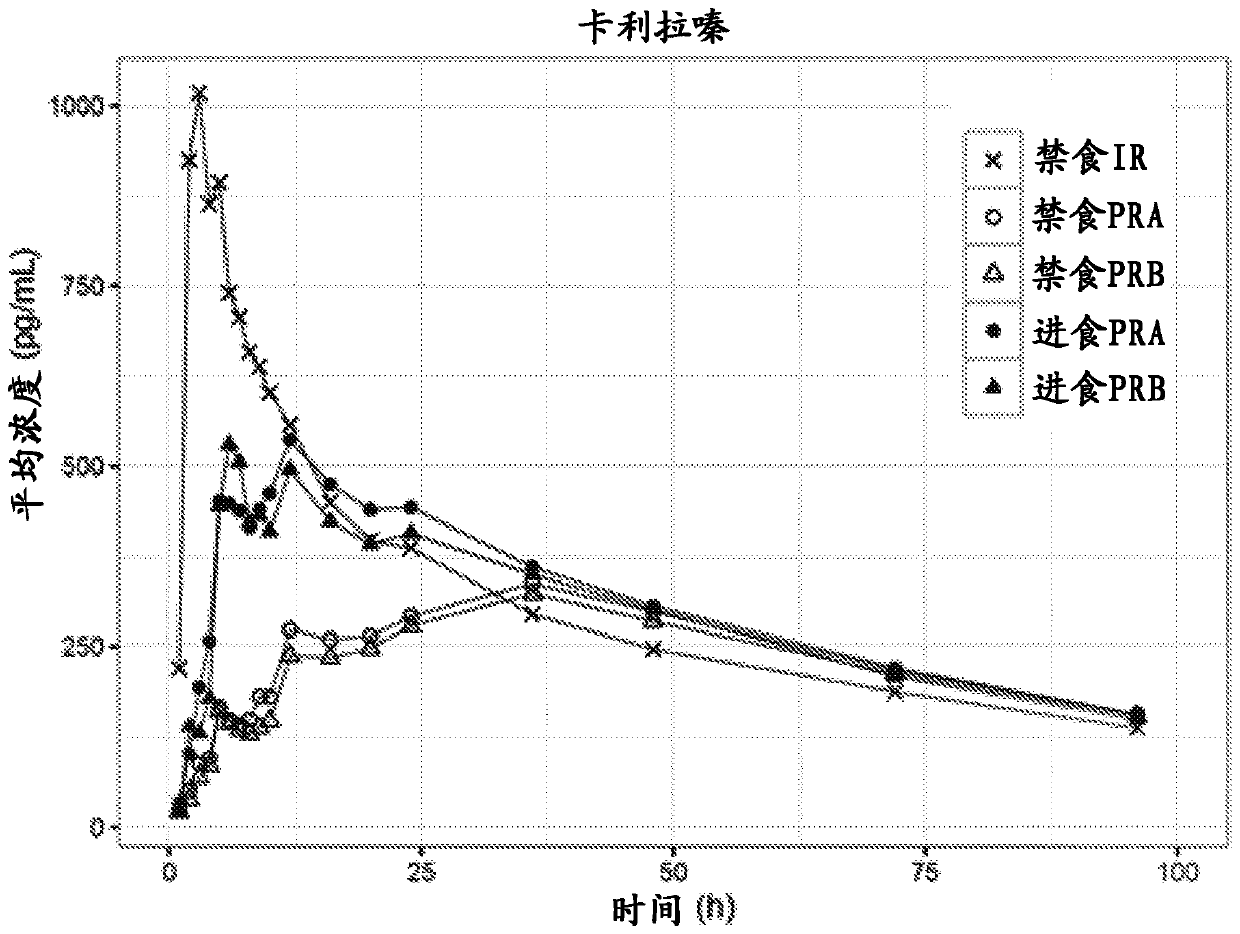
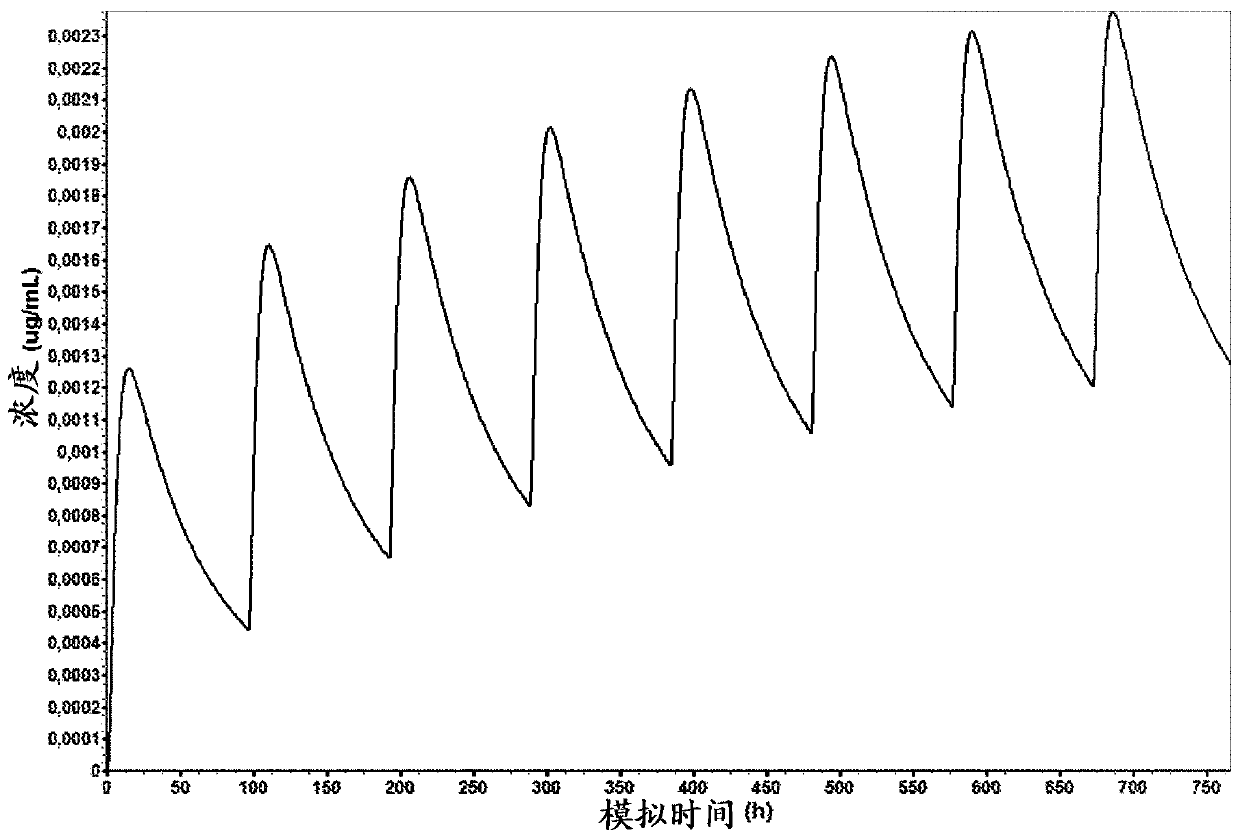
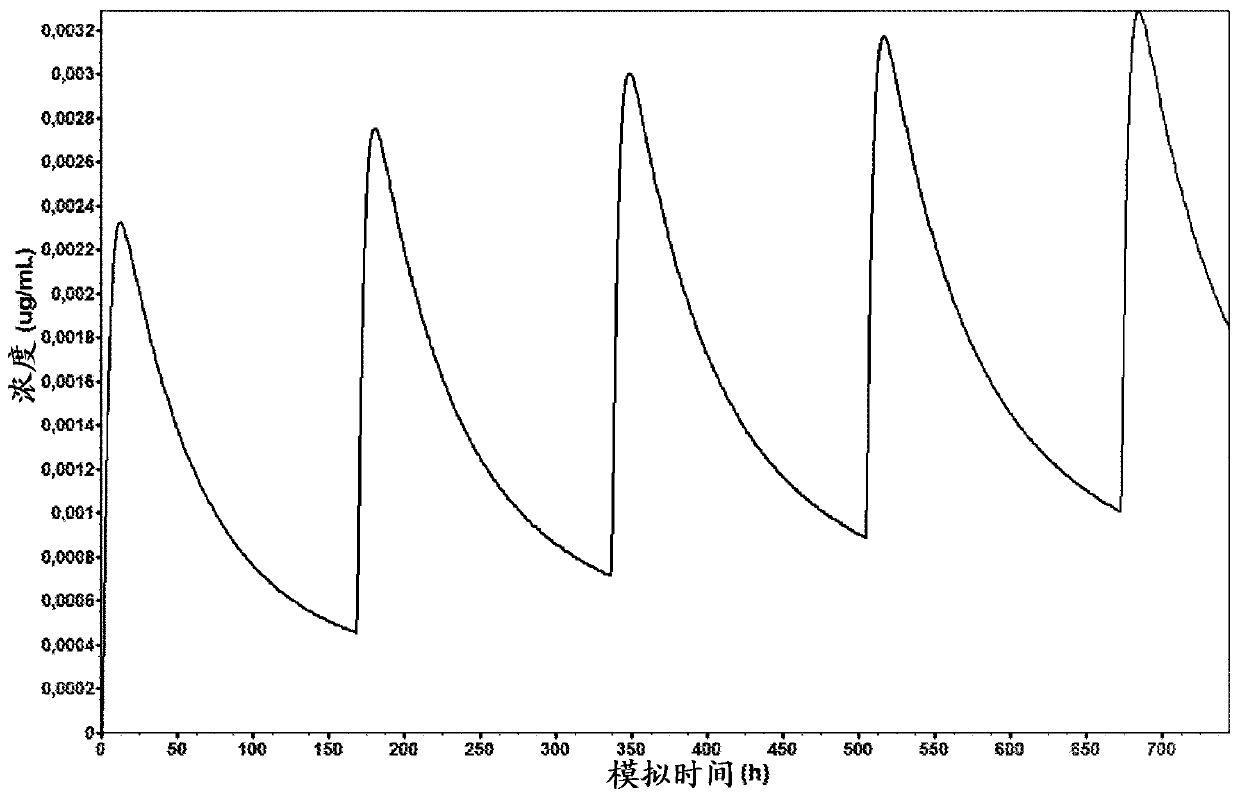
Solid preparation of cariprazine for oral administration
A technology of cariprazine and modified-release cariprazine, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., and can solve the problem of drug insolubility in neutral or alkaline environments And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Embodiment 1: floating sheet (F1)
[0215] F1 floating tablets were prepared by fluidized granulation, in which cariprazine was mixed with microcrystalline cellulose and alginic acid in a fluidized bed apparatus; the mixture was then sprayed with aqueous citric acid. The dried granules were covered with glyceryl distearate by heating the granules. In the final step, the granules are mixed with the external phase (hypromellose, sodium bicarbonate, colloidal anhydrous silicon dioxide, magnesium stearate) and compressed into tablets using rotary tablet press equipment.
[0216] The composition contains gas forming agents and release modifiers to increase residence time in the stomach throughout the 8 hour dissolution time course.
[0217]
[0218] Table 4: Qualitative and quantitative composition of F1 floating flakes
[0219] F1 floating tablets exhibit an in vitro release profile in which, on average, no more than about 15-35% of the total cariprazine is released ...
Embodiment 2
[0223] Embodiment 2: floating sheet (F2)
[0224] F2 floating tablets were prepared by fluidized granulation, in which cariprazine was mixed with microcrystalline cellulose and alginic acid in a fluidized bed apparatus; the mixture was then sprayed with an aqueous solution of citric acid. The dried granules were covered with glyceryl distearate by heating the granules. In the final step, the granules are mixed with the external phase (lactose monohydrate, hypromellose, sodium bicarbonate, colloidal anhydrous silicon dioxide, magnesium stearate) and compressed into tablets using rotary tablet press equipment agent.
[0225] The composition contains gas forming agents and release modifiers to increase residence time in the stomach throughout the 8 hour dissolution time course.
[0226]
[0227] Table 6: Qualitative and quantitative composition of F2 floating flakes
[0228] F2 floating tablets exhibit an in vitro release profile in which, on average, no more than about 2...
Embodiment 3
[0232] Example 3: Capsules (F3) Containing Bioadhesive Beads
[0233] The F3 capsule composition was prepared by the following steps: mixing cariprazine with microcrystalline cellulose and polyacrylic acid polymer in a high shear mixer; after granulating with liquid, extruding the granulated mixture to form a suitable Cylindrical aggregates, which are then rounded into round pellets. Before encapsulation, the pellets are dried in fluid bed equipment; the pellets are sieved to target size and lubricated with talc and calcium stearate. The resulting pellets are filled into hard gelatin capsules.
[0234]
[0235] Table 8: Qualitative and quantitative composition of F3 bioadhesive beads for capsules
[0236] F3 capsules exhibit an in vitro release profile in which, on average, no more than about 55-65% of the total cariprazine is released within 1 hour and no more than the total cariprazine is released within 3 hours after placement in a standard dissolution testing appara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com