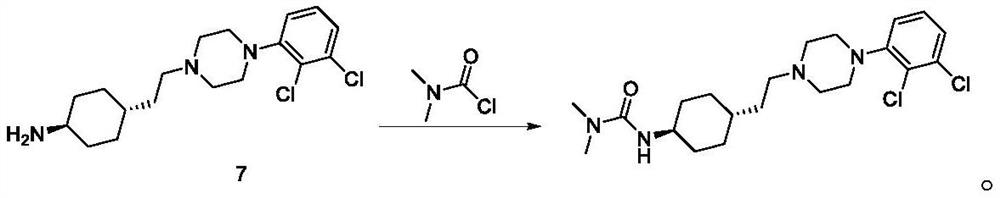
Preparation method of cariprazine and intermediate thereof
An intermediate, volumetric technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of unsuitable for industrial production, strong equipment corrosion, cumbersome reaction steps, etc., to achieve safe and controllable drug quality and high reaction yield. , the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
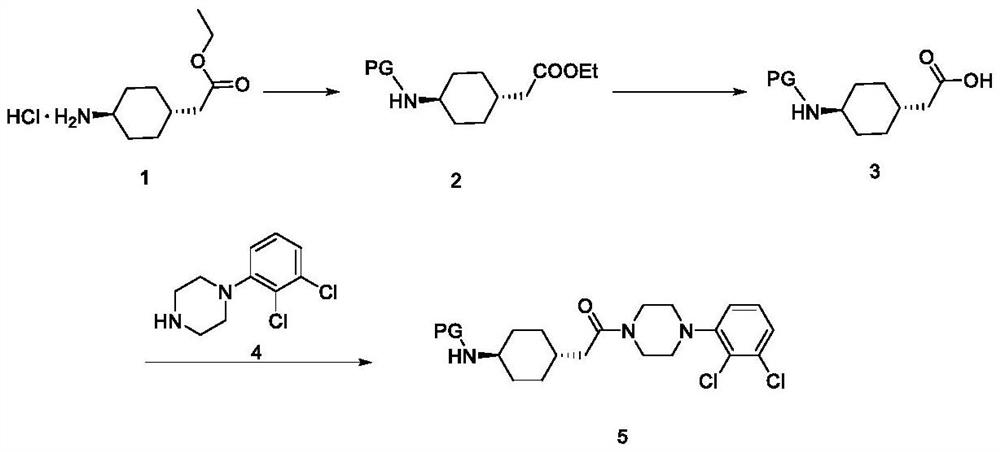
[0079] Example 1 Preparation of trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl acetate (cariprazine intermediate 2)
[0080] In a 50mL there-necked flask, add 4g (0.018mol) of trans-2-[1-(4-amino]-cyclohexyl)-ethyl acetate hydrochloride 1 and 16mL of dichloromethane, then add 1.8g (0.018mol) of Triethylamine, the mixture was cooled to 5-10 ℃, and then under nitrogen protection, 4.0 g (0.018 mol) of di(tert-butyl) dicarbonate solution in 10 mL of dichloromethane was added dropwise, and stirred at 20 ℃ after the dropwise addition. , After 10h, the reaction was completed, 20mL of saturated sodium bicarbonate solution was added, and the phases were separated. The organic layer was washed with 10 mL of water, and the phases were separated with anhydrous Na 2 SO 4 The organic phase was dried, the solvent was removed under reduced pressure, and dried under reduced pressure at 30° C. to obtain 4.64 g of the target compound with a yield of 90.0%. HPLC purity 98.5%. ...
Embodiment 2
[0081] Example 2 Preparation of trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-acetic acid (cariprazine intermediate 3)
[0082] In a 50mL three-necked flask, add 4.0g (0.014mol) of trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl acetate (cariprazine intermediate 2 ), 40 mL of tetrahydrofuran, 10 mL of distilled water and 1.18 g (0.028 mol) of lithium hydroxide monohydrate, and the reaction was stirred at 40 °C for 6 h. After the reaction was completed, the tetrahydrofuran was removed under reduced pressure, 14 mL (6 M) of hydrochloric acid was added for acidification, and after stirring in an ice bath for 1 h, Filtration, washing with 10 mL of distilled water, drying under reduced pressure at 50° C. to obtain 1.8 g of the target compound with a yield of 90.0% and HPLC purity of 99.0%.
Embodiment 3
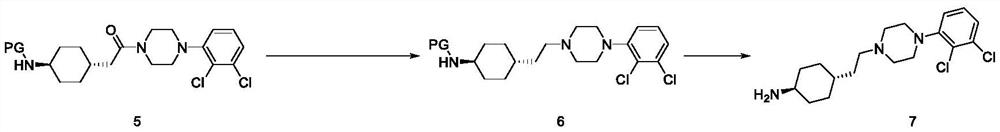
[0083] Example 3 tert-butyl (1R,4R-4-(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)-2-oxoethyl)cyclohexyl)carbamate Preparation of (Cariprazine Intermediate 5)
[0084] Method a: In a 50mL three-necked flask, add 3.86g (0.015mol) of trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-acetic acid (cariprazine intermediate body 3), 20 mL of acetone and 2.9 g (0.018 mol) of N,N-carbonyldiimidazole, stirred at 25 °C for 4 h, added 2 mL of isopropanol, stirred at 25 °C for 1 h, then added 2.1 mL of triethylamine and 3.47 g (0.015 mol of ) 1-(2,3-dichlorophenyl)-piperazine 4, stirred at room temperature for 12 hours, after the reaction was completed, added 80 mL of distilled water, stirred for 1 hour, filtered, and washed with 40 mL of distilled water. Drying under reduced pressure at 45° C. obtained 6.4 g of the target compound with a yield of 90.0% and HPLC purity of 99.5%.
[0085] Method b: add 5 g (0.019 mol) of trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-acetic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com