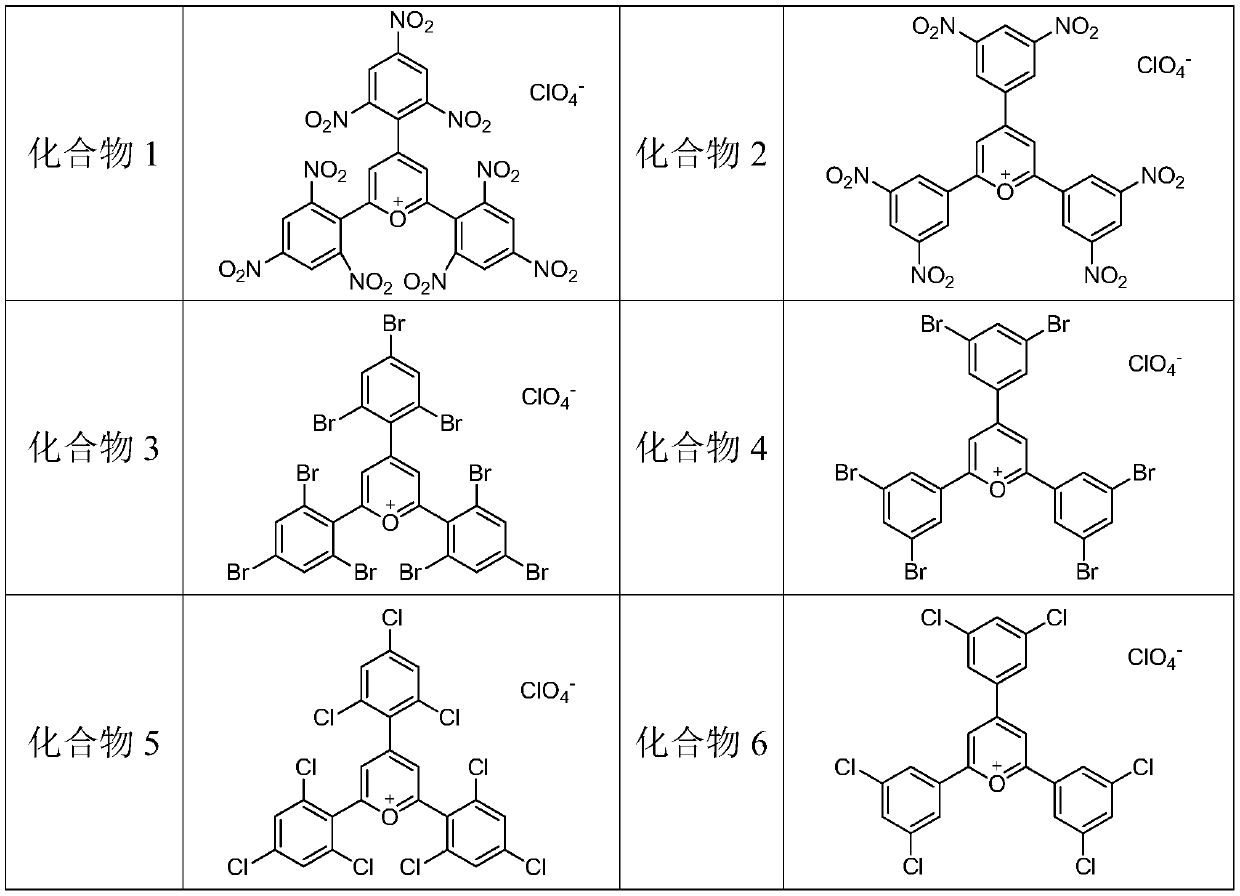
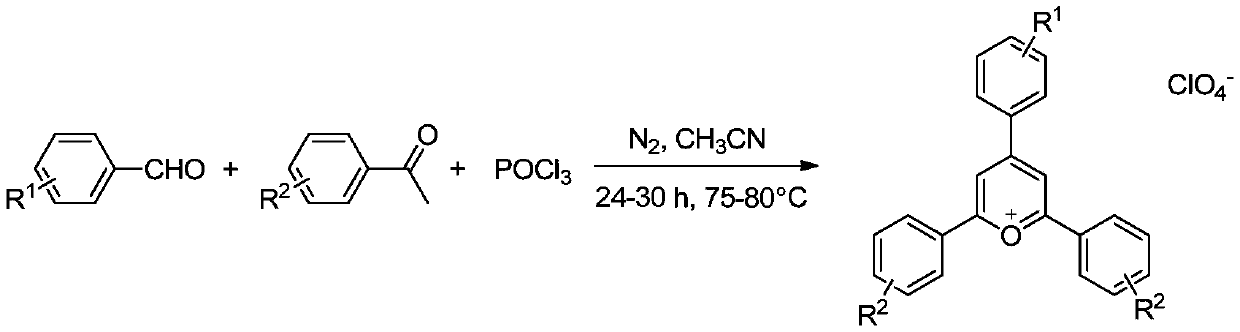
Ag-C co-doped TiO2-perchloric acid pyranium salt photocatalyst and preparation method thereof
A technology of perchloric acid pyranium salt and photocatalyst, which is applied in the field of Ag-C co-doped TiO2-perchloric acid pyranium salt photocatalyst, can solve the difficulty of recycling organic dye-based photocatalysts, low redox potential, and photocatalysts. problems such as poor catalytic performance, to achieve the effect of improving practicability and service life, increasing catalytic activity, and high oxidation potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of Ag-C co-doped TiO2 includes the following steps: add 200-500mL of absolute ethanol to the hydrothermal synthesis automatic reaction kettle, add 3-5 parts of silver nitrate and stir to dissolve at a constant speed, and then add 70-82 parts of titanic acid in sequence After isopropyl ester, 10-15 parts of glucose and 5-10 parts of cetyltrimethylammonium bromide are stirred and dissolved at a constant speed, then slowly add dilute hydrochloric acid drop by drop to adjust the pH of the solution to 3-4, and set the temperature of the automatic reactor to 120 -130°C, stirring at a constant speed for 5-7h. After the reaction, the material is concentrated under reduced pressure to remove ethanol, and the solid mixture is placed in a tubular resistance furnace. The heating rate is 5°C / min, and the temperature is raised to 520-550°C and maintained at the temperature. Calcined for 4-6 hours, put the calcined product in a high-energy planetary ball mill, se...
Embodiment 1
[0031] (1) Preparation of Ag-C co-doped TiO The preparation method comprises the following steps: add 200mL of absolute ethanol to the hydrothermal synthesis automatic reaction kettle, add 3 parts of silver nitrate and stir and dissolve at a uniform speed, then add 82 parts of isopropyl titanate successively After the ester, 10 parts of glucose and 5 parts of cetyltrimethylammonium bromide were stirred and dissolved at a constant speed, dilute hydrochloric acid was slowly added dropwise to adjust the pH of the solution to 3, and the temperature of the automatic reactor was set to 120°C, and the reaction was stirred for 5 hours at a constant speed. After the end, the material was concentrated under reduced pressure to remove ethanol, and the solid mixture was placed in a tubular resistance furnace at a heating rate of 5°C / min, raised to 520°C and maintained at the temperature for calcination for 4 hours, and the calcined product was placed in a high-energy planetary ball mill, se...
Embodiment 2
[0036] (1) Preparation of Ag-C co-doped TiO The preparation method comprises the following steps: add 200mL of absolute ethanol to the hydrothermal synthesis automatic reaction kettle, add 3 parts of silver nitrate and stir and dissolve at a uniform speed, then add 82 parts of isopropyl titanate successively After the ester, 10 parts of glucose and 5 parts of cetyltrimethylammonium bromide were stirred and dissolved at a constant speed, dilute hydrochloric acid was slowly added dropwise to adjust the pH of the solution to 3, the temperature of the automatic reactor was set to 130°C, and the reaction was stirred for 5 hours at a constant speed. After the end, the material was concentrated under reduced pressure to remove ethanol, and the solid mixture was placed in a tubular resistance furnace at a heating rate of 5°C / min, raised to 520°C and maintained at the temperature for calcination for 4 hours, and the calcined product was placed in a high-energy planetary ball mill, set T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com