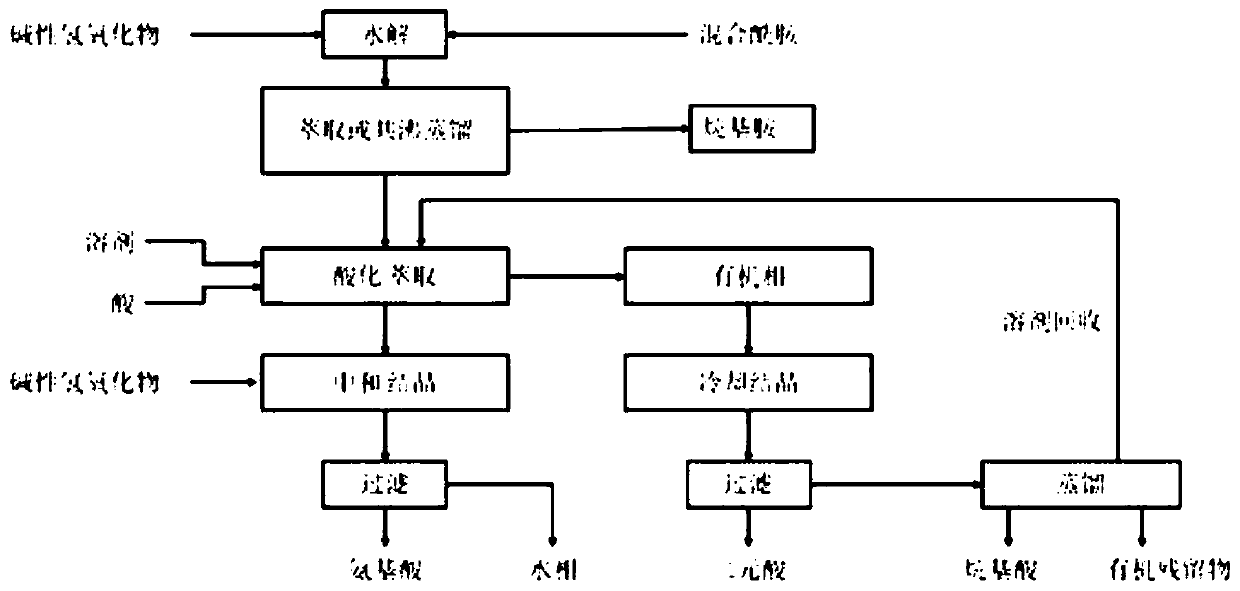
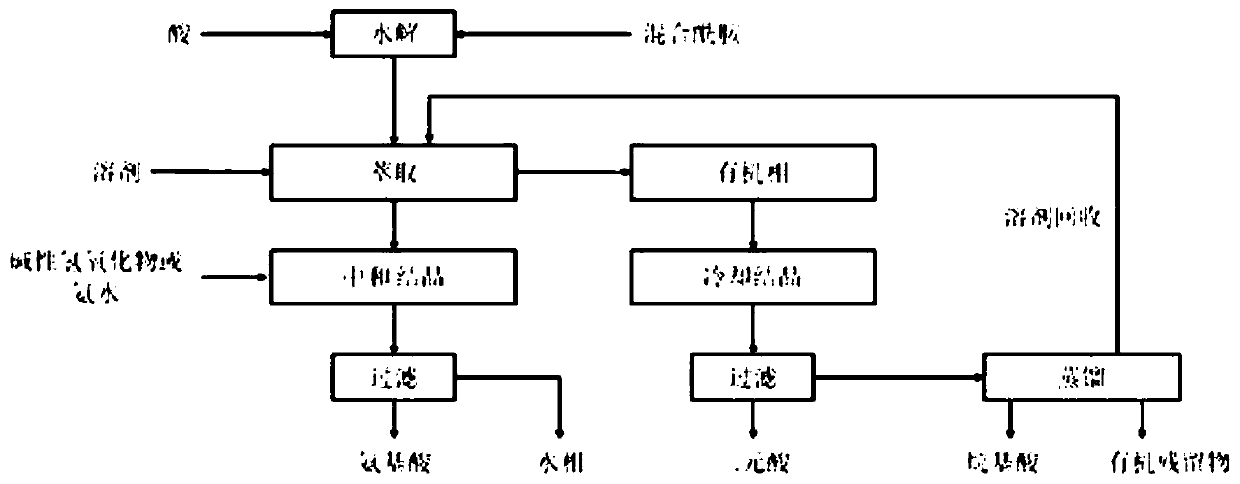
Process for the separation of long chain amino acids and dibasic acids
A long-chain dibasic acid and amino acid technology, applied in separation methods, evaporation separation crystallization, purification/separation of amino compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This example concerns the separation of 11-aminoundecanoic acid, dodecanedioic acid, hexylamine, heptanoic acid and stearic acid from a mixture obtained by hydrolysis of mixed amide derivatives with sodium hydroxide.
[0080]According to WO2017 / 088218, 150 g of mixed amide derivatives were prepared from methyl 12-carbonyl stearate, and then added to 60 g of sodium hydroxide and 800 mL of water for hydrolysis to obtain a mixture of starting solutions.
[0081] The solution was azeotropically distilled with a 2.5×30 cm vacuum jacketed column filled with ceramic saddle packing. First, methanol is obtained, and then the azeotrope of hexylamine and water is distilled until the pH of the upper fraction is neutral and the pH value is 7-8. The fraction separates into two phases, with the lower aqueous phase being continuously returned to the distillation flask. The crude hexylamine was dehydrated by azeotropic distillation to obtain 20.5 g of hexylamine.
[0082] To the remai...
Embodiment 2
[0089] This example concerns the separation of 11-aminoundecanoic acid, dodecanedioic acid, hexylamine, heptanoic acid and stearic acid from a mixture obtained by sulfuric acid hydrolysis of mixed amide derivatives.
[0090] According to WO2017 / 088218, 150 g of mixed amide derivatives were prepared from 12-carbonyl stearic acid methyl ester, and then added to 150 g of sulfuric acid and 30 g of water for hydrolysis to obtain a mixture of initial suspensions. During the hydrolysis, low-boiling methanol has been continuously removed.
[0091] 800 g of water and 800 mL of toluene were added to the reaction suspension. The mixture was vigorously stirred at 85 °C for 60 min and then transferred to a separatory funnel for phase separation.
[0092] Using the same treatment method as in Example 1 to treat the toluene phase, each component obtained similar results.
[0093] The aqueous phase was neutralized to a neutral pH of 7.5 with aqueous sodium hydroxide solution, consuming a to...
Embodiment 3
[0097] This example concerns the separation of 9-aminononanoic acid, sebacic acid, octylamine and nonanoic acid.
[0098] According to WO2017 / 088218, 150 g of mixed amide derivatives were prepared from methyl 10-carbonyl stearate, and then added to 60 g of sodium hydroxide and 800 mL of water for hydrolysis to obtain a mixture of starting solutions.
[0099] To the cloudy solution was added 200 mL of toluene and stirred vigorously at 80°C for 45 minutes. Then the toluene phase was separated and the toluene was recovered, and the remaining residue continued to be distilled to obtain 29.5 g of n-octylamine.
[0100] The aqueous phase was treated in the same manner as in Example 1 to obtain 36.9 g of nonanoic acid, 39.6 g of 9-aminononanoic acid and 45.5 g of sebacic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com