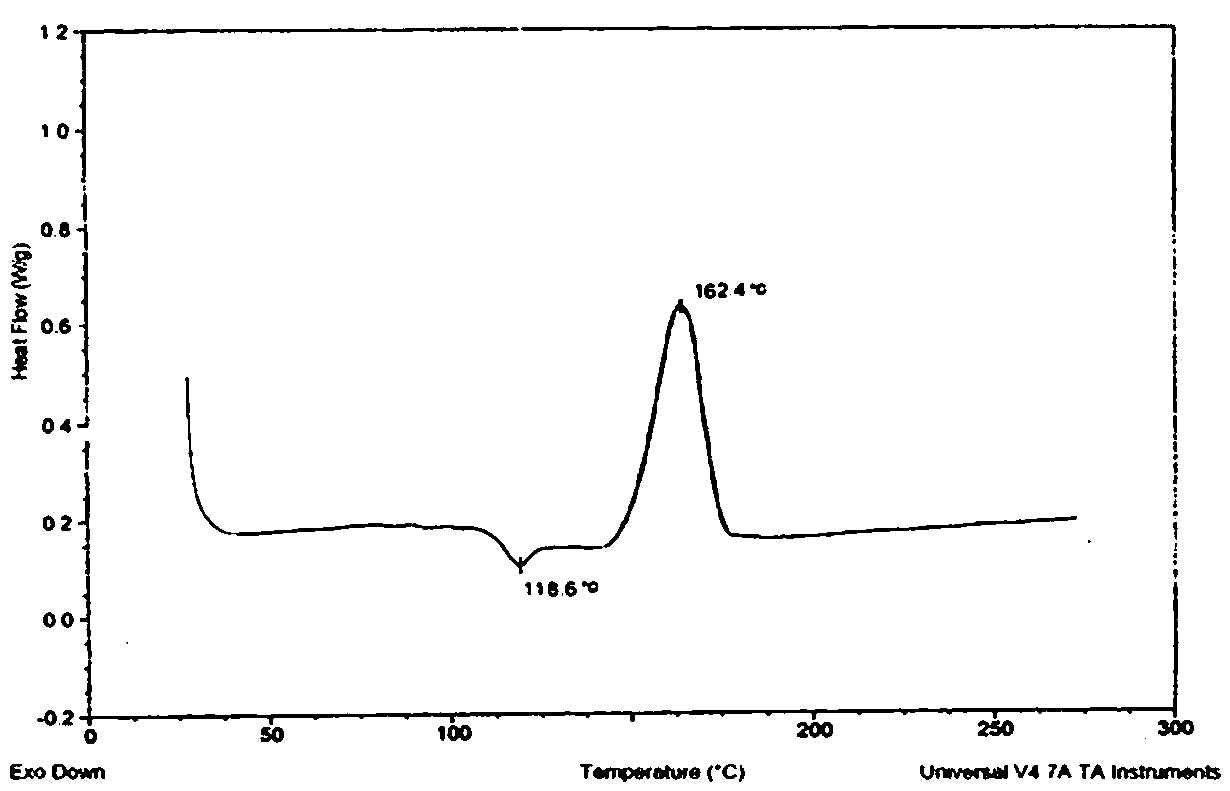
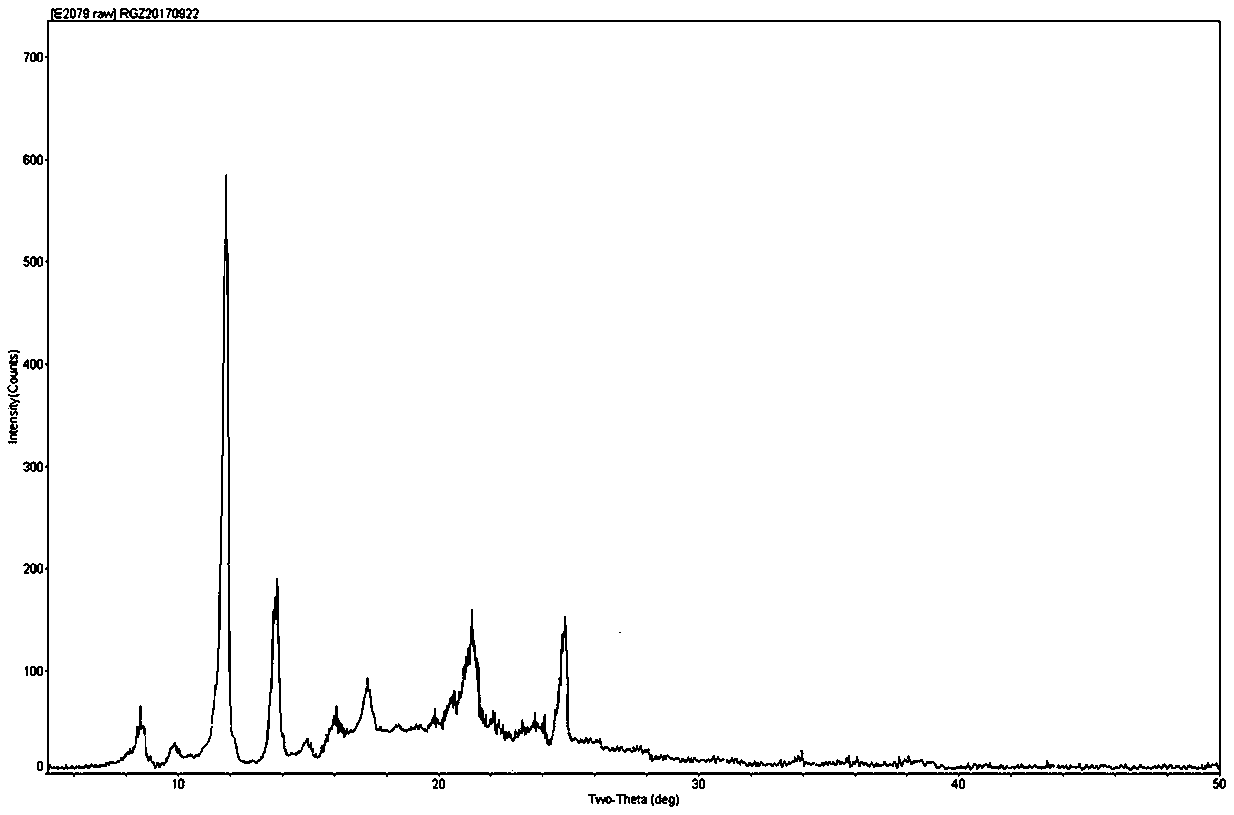
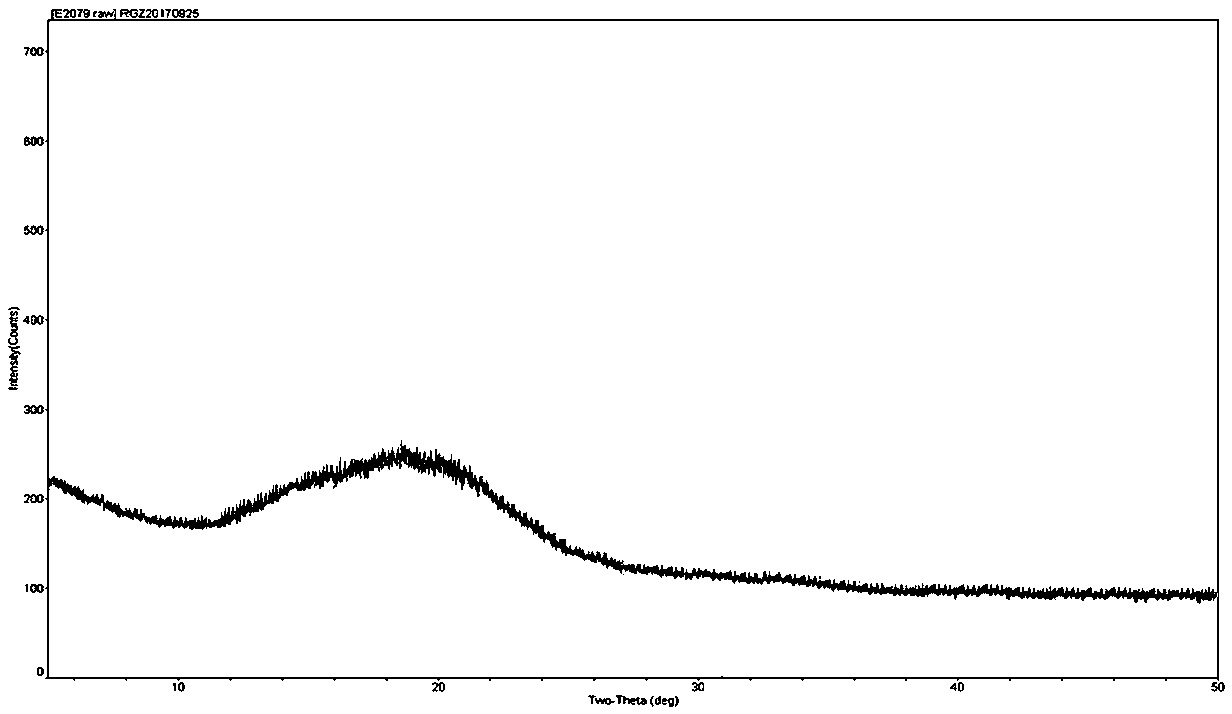
Colchicine compound, preparation method, preparation, applications and pharmaceutical composition thereof
A technology of colchicine and compounds, applied in the direction of drug combination, organic chemical methods, antipyretics, etc., to achieve the effects of improved bioavailability, high product yield and purity, and improved safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of colchicine compound of the present invention comprises the following steps:
[0049] A, heating and dissolving colchicine in a solvent, and filtering to obtain filtrate a;
[0050] B. Cool the filtrate a to -40°C~-30°C and pre-freeze for 3~5h, then sublimate and dry to obtain the target colchicine compound.
[0051] The colchicine in the step A is heated and dissolved at a temperature of 60-80° C., and the heating time is 0.5-2 hours.
[0052] The mass ratio of colchicine and solvent in step A is (0.01-50):100.
[0053] Described solvent is water, methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, ethylene glycol, propylene glycol, glycerol, acetone, ethyl acetate, dimethyl sulfoxide, N , One or more of N-dimethylformamide.
[0054] The sublimation drying described in step B is to control the temperature at -25°C~-20°C and the pressure 10~20Pa for 3~5h.
[0055] The analytical drying described in step B is to control the tem...
Embodiment 1
[0082] Example 1 Preparation of Amorphous Colchicine Compound 1# Sample
[0083] Weigh 0.5 g of colchicine, dissolve it in 100 g of water, heat and dissolve at 70-80°C for 1 hour, filter, put the filtrate in a vacuum freeze dryer, cool down to -40~-30°C, and pre-freeze for 3~5 hour, then control temperature -25~-20℃, pressure 10~20Pa, sublimation drying for 3~5 hours, finally control temperature 30~50℃, pressure 1~10Pa, analysis drying for 4~6 hours, that is.
Embodiment 2
[0084] Example 2 Preparation of Amorphous Colchicine Compound 2# Sample
[0085] Weigh 0.5 g of colchicine, dissolve in 10 g of methanol, heat and dissolve at 70-80°C for 1 hour, filter, put the filtrate in a vacuum freeze dryer, cool down to -40~-30°C, and pre-freeze for 3~ 5 hours, then control temperature -25~-20℃, pressure 10~20Pa, sublimation drying for 3~5 hours, finally control temperature 30~50℃, pressure 1~10Pa, analysis and drying for 4~6 hours, that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com