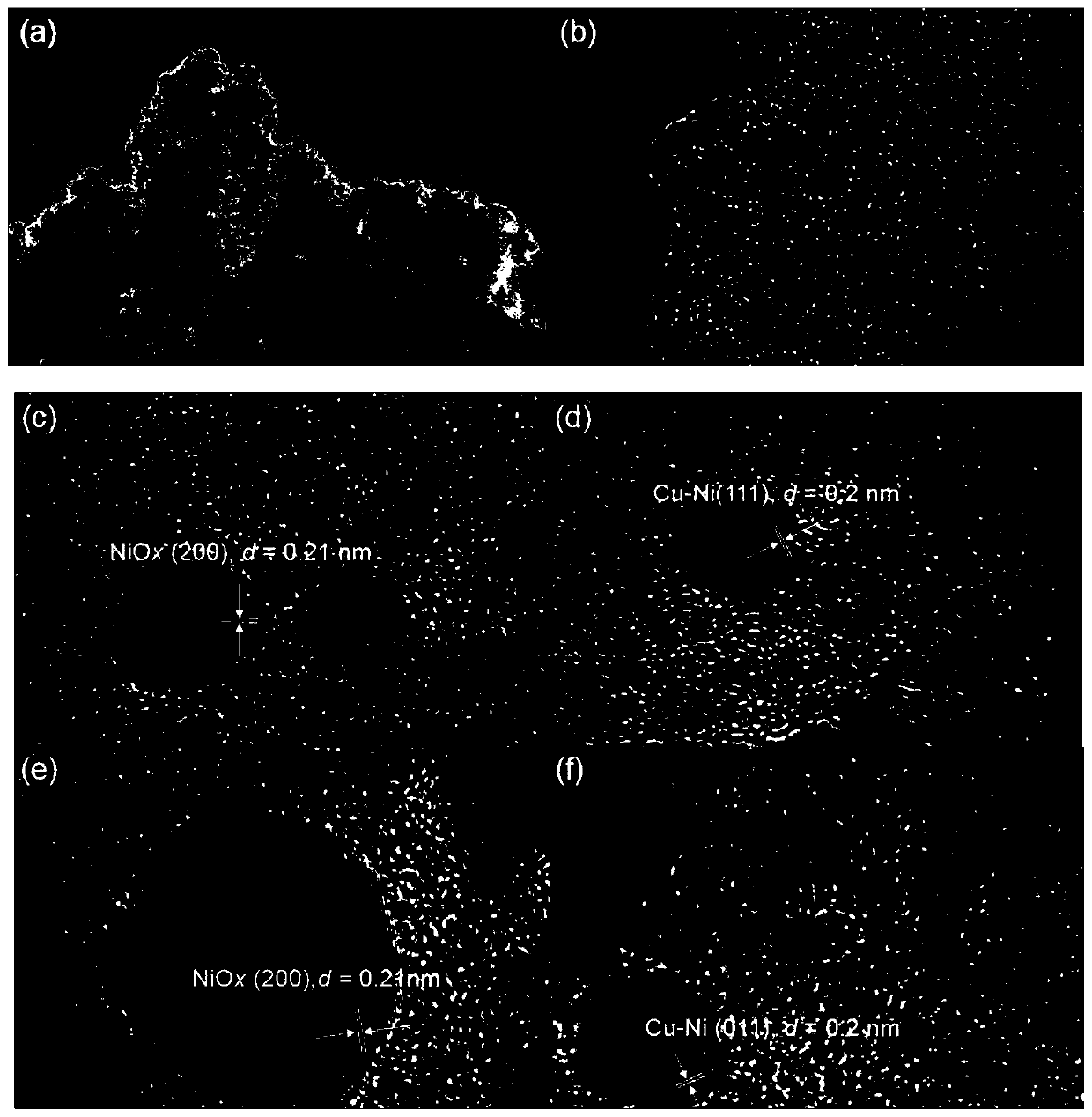
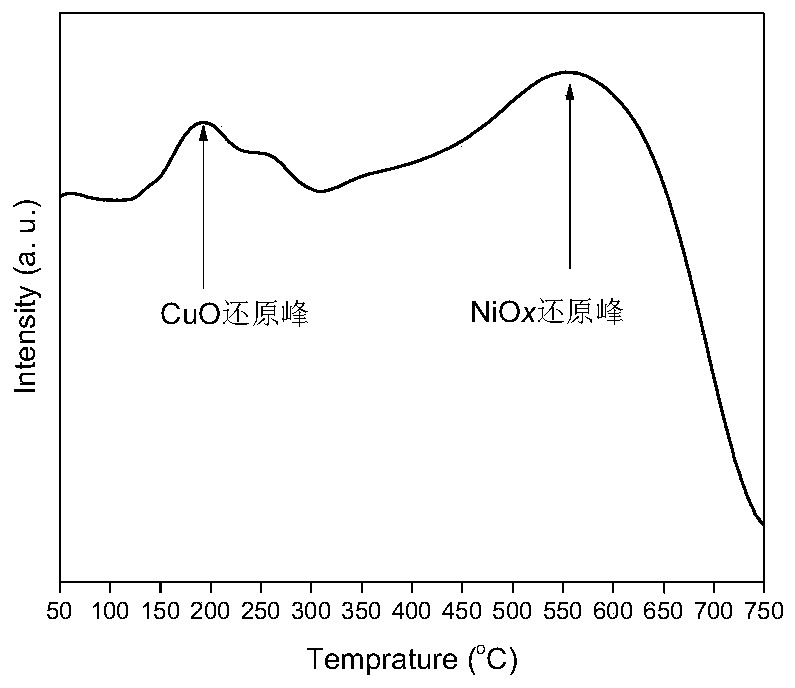
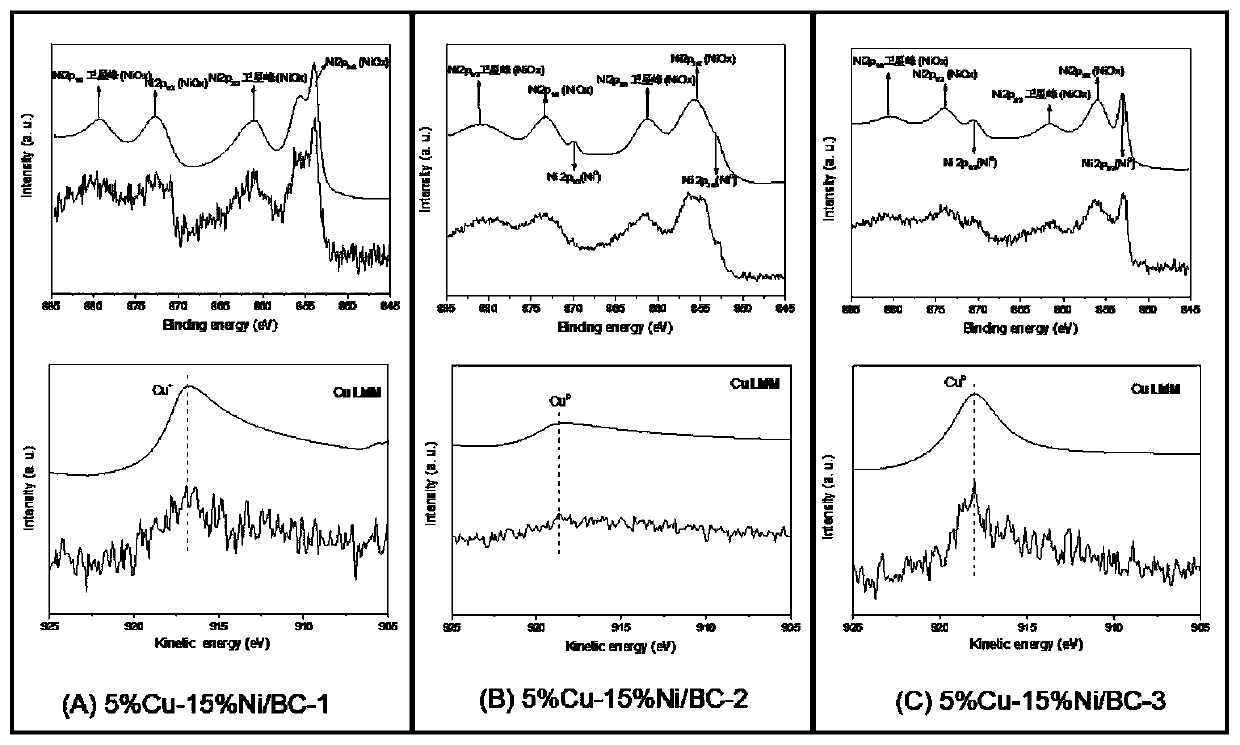
Cu-Ni bimetallic catalyst with charcoal being carrier and application of Cu-Ni bimetallic catalyst
A bimetallic catalyst and biochar technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of few reports on catalyst carrier and achieve cheap raw materials , saving resources and protecting the natural environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of biochar is the Cu-Ni bimetallic catalyst of carrier, is prepared by following steps:
[0031] (1) Under magnetic bar stirring, 2.0 g of α-cellulose and 20.0 g of 1-butyl-3-methylimidazolium chloride ([BMim]Cl) were mixed in a flask, and then the mixture was placed in an oil bath While heating in medium temperature, add 12.0 mL of concentrated H2 dropwise with an acid burette 2 SO 4 Until the temperature rose to 150° C., after 15 h, the prepared sample was filtered through a millipore filter (0.22 μm pore size), and washed with deionized water until the filtrate was neutral. Finally, the samples were vacuum-dried overnight at 60 °C to obtain biochar precursors.
[0032] (2) In a tube furnace, the biochar precursor obtained in step (1) was carbonized at 600 °C for 3 h (heating rate 3 °C / min) by nitrogen gas flow (purity ≥ 99.9%), and then heated at room temperature with The pyrolyzed biochar was washed with ethanol to remove the tar generated during pyrolysis...
Embodiment 2
[0046] A kind of biochar is the Cu-Ni bimetallic catalyst of carrier, is prepared by following steps:
[0047] (1) Mix 3.0 g of α-cellulose with 20.0 g of 1-butyl-3-methylimidazolium chloride ([BMim]Cl) in a flask under stirring with a magnetic bar, and then place the mixture in an oil bath While heating in medium temperature, add 18.0 mL of concentrated H2 dropwise with an acid burette 2 SO 4 After the temperature rose to 150° C., after 15 h of reaction, the prepared sample was filtered through a millipore filter (0.22 μm pore size), and washed with deionized water until the filtrate was neutral. Finally, the samples were vacuum-dried overnight at 60 °C to obtain biochar precursors.
[0048] (2) In a tube furnace, the biochar precursor obtained in step (1) was carbonized at 500 °C for 4 h (heating rate was 3 °C / min) through nitrogen gas flow (purity ≥ 99.9%), and then heated at room temperature with The pyrolyzed biochar was washed with ethanol to remove the tar generated ...
Embodiment 3
[0051] A kind of biochar is the Cu-Ni bimetallic catalyst of carrier, is prepared by following steps:
[0052] (1) Under magnetic bar stirring, 2.0 g of α-cellulose and 20.0 g of 1-butyl-3-methylimidazolium chloride ([BMim]Cl) were mixed in a flask, and then the mixture was placed in an oil bath While heating in medium temperature, add 12.0 mL of concentrated H2 dropwise with an acid burette 2 SO 4 After the temperature rose to 160° C., after 15 h of reaction, the prepared sample was filtered through a millipore filter (0.22 μm pore size), and washed with deionized water until the filtrate was neutral. Finally, the samples were vacuum-dried overnight at 60 °C to obtain biochar precursors.
[0053] (2) In a tube furnace, the biochar precursor obtained in step (1) was carbonized at 700 °C for 3 h (heating rate was 3 °C / min) through nitrogen gas flow (purity ≥ 99.9%), and then heated at room temperature with The pyrolyzed biochar was washed with ethanol to remove the tar gener...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com