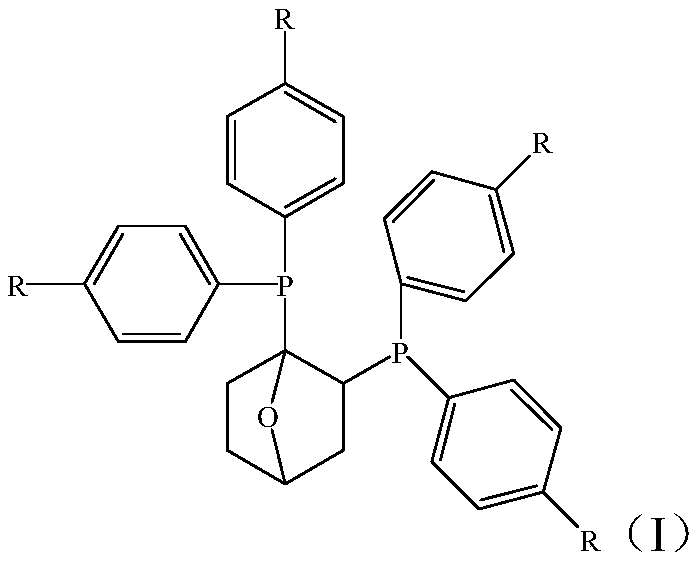
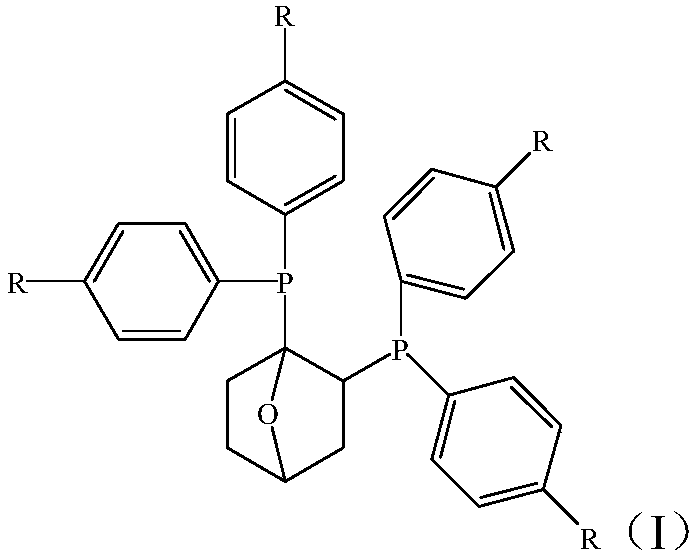
Ethylene tetramer catalyst composition and application thereof
A technology of ethylene tetramerization and catalyst, which is applied in the direction of catalytic reaction, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc. It can solve the problems of poor yield and low selectivity of 1-octene , to achieve high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A 500mL autoclave was used to vacuumize and replace with nitrogen for 3 times, then filled with ethylene for 2 times, and lowered to room temperature. Then dry anhydrous toluene was added at 30°C, and 20 μmol (1,2)-bis(diphenylphosphine)-[2,2,1]-O-cyclohexane, chromium acetylacetonate and activator triethyl base aluminum, the total volume of the composition is 100mL, wherein the molar ratio of oxygen-containing bridged cyclohexane ligand compound, chromium salt and activator triethylaluminum is 1:0.5:300, the reaction pressure is controlled at 2.0MPa, and ethylene , heated up to 60°C for ethylene tetramerization. After the reaction was completed, the system was cooled to room temperature, the gas phase product was collected in a gas metering tank, and the liquid phase product was collected in an Erlenmeyer flask, and 1 mL of ethanol was added as a terminator to terminate the ethylene tetramerization reaction. The test is carried out, and the test data results are shown...
Embodiment 2
[0032] A 500mL autoclave was used to vacuumize and replace with nitrogen for 3 times, then filled with ethylene for 2 times, and lowered to room temperature. Then dry anhydrous toluene was added at 30°C, and 20 μmol of (1,2)-bis(diphenylphosphine)-[2,2,1]-O-cyclohexane, tris(tetrahydrofuran)chromium trichloride were added simultaneously and activator triethylaluminum, the total volume of the composition is 100mL, wherein the molar ratio of oxygen bridged cyclohexane ligand compound, chromium salt and activator triethylaluminum is 1:0.5:300, and the reaction pressure is controlled to 2.0 MPa, feed ethylene, heat up to 60°C, and carry out ethylene tetramerization reaction. After the reaction was completed, the system was cooled to room temperature, the gas phase product was collected in a gas metering tank, and the liquid phase product was collected in an Erlenmeyer flask, and 1 mL of ethanol was added as a terminator to terminate the ethylene tetramerization reaction. The test...
Embodiment 3
[0034] A 500mL autoclave was used to vacuumize and replace with nitrogen for 3 times, then filled with ethylene for 2 times, and lowered to room temperature. Then dry anhydrous toluene was added at 30°C, and 20 μmol (1,2)-bis(diphenylphosphine)-[2,2,1]-O-cyclohexane, chromium trichloride and activator three were added at the same time. Ethylaluminum, the total volume of the composition is 100mL, wherein the molar ratio of oxygen bridged cyclohexane ligand compound, chromium salt and activator triethylaluminum is 1:0.5:300, the reaction pressure is controlled at 2.0MPa, and the Ethylene, the temperature is raised to 60°C to carry out ethylene tetramerization reaction. After the reaction was completed, the system was cooled to room temperature, the gas phase product was collected in a gas metering tank, and the liquid phase product was collected in an Erlenmeyer flask, and 1 mL of ethanol was added as a terminator to terminate the ethylene tetramerization reaction. The test is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com