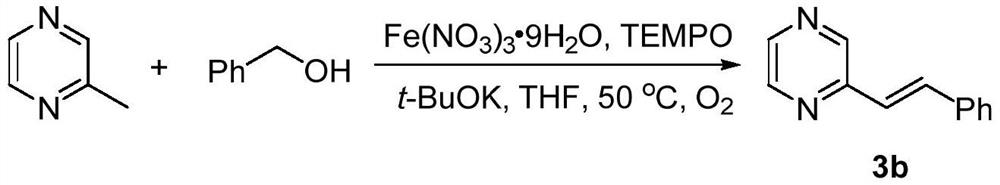A kind of preparation method of trans-type disubstituted olefin
A double-substituted, olefinic technology, applied in the direction of organic chemistry, can solve problems such as a large amount of metal waste, expensive catalysts and high-temperature reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
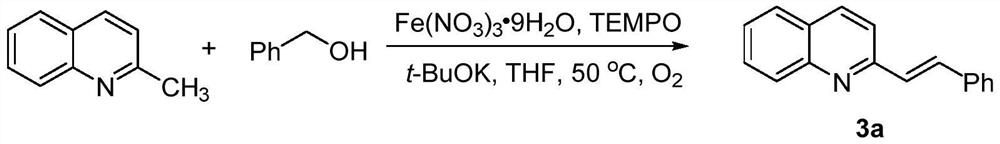
[0031] This example provides a preparation method of trans-disubstituted olefin 3a:
[0032] Its synthetic route is:
[0033]
[0034] 2-Methylquinoline (1mmol), benzyl alcohol (2.5mmol), TEMPO (0.1mmol), Fe(NO 3 ) 3 9H 2 O (0.1 mmol) and t-BuOK (2 mmol) were sequentially added to a flask containing 2 mL of THF, the air in the flask was replaced with oxygen, then stirred at 50°C, and the reaction process was monitored by thin-layer chromatography. After the reaction was complete, it was quenched with water (10 mL).
[0035] The reaction product was extracted with ethyl acetate (3×20 mL), washed with saturated brine (20 mL), washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the obtained filtrate, 196.6 mg was obtained by silica gel column chromatography (200-300 mesh, the developing solvent was a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1), and the yield was 85%.
[0036] Gained product is carried out nuclea...
Embodiment 2
[0039] This example provides a preparation method of trans disubstituted olefin 3b:
[0040] The synthetic route is:
[0041]
[0042] 2-methylpyrazine (1mmol), benzyl alcohol (2.5mmol), TEMPO (0.1mmol), Fe(NO 3 ) 3 9H 2 O (0.1 mmol) and t-BuOK (2 mmol) were sequentially added to a flask containing 2 mL of THF, the air in the flask was replaced with oxygen, then stirred at 50°C, and the reaction process was monitored by thin-layer chromatography. After the reaction was complete, it was quenched with water (10 mL).
[0043] The reaction product was extracted with ethyl acetate (3×20 mL), washed with saturated brine (20 mL), washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the obtained filtrate, 136.7 mg of the product was obtained by silica gel column chromatography (200-300 mesh, the developing solvent was a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 15:1), and the yield was 75%.
[0044] Gained product is carr...
Embodiment 3
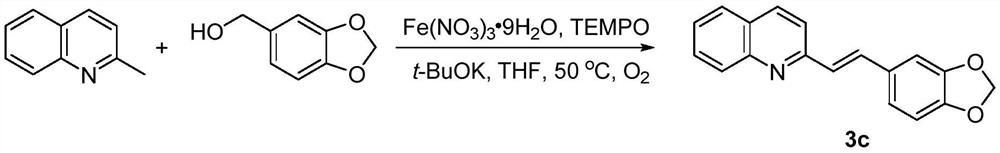
[0047] This example provides a preparation method of trans disubstituted olefin 3c:
[0048] Its synthetic route is:
[0049]
[0050] 2-Methylquinoline (1mmol), piperonyl alcohol (2.5mmol), TEMPO (0.1mmol), Fe(NO 3 ) 3 9H 2 O (0.1 mmol) and t-BuOK (2 mmol) were sequentially added to a flask containing 2 mL of THF, the air in the flask was replaced with oxygen, then stirred at 50°C, and the reaction process was monitored by thin-layer chromatography. After the reaction was complete, it was quenched with water (10 mL).
[0051] The reaction product was extracted with ethyl acetate (3×20 mL), washed with saturated brine (20 mL), washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the obtained filtrate, 123.9 mg of the product was obtained by silica gel column chromatography (200-300 mesh, the developing solvent was a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 20:1), and the yield was 45%.
[0052] Gained product is ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com